MBI Videos
Workshop 1: Host-Pathogen Dynamics
-
 Malidi Ahamadi
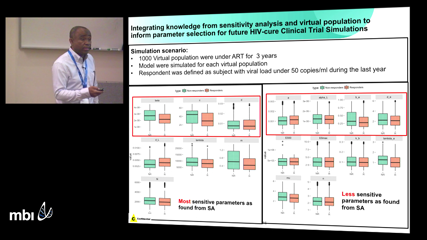
Malidi AhamadiAbstract not submitted.
-
 Ioannis (Yannis) AndroulakisIn this talk I will discuss key ideas emphasizing the central role circadian dynamics, and their misalignment, play in orchestrating and coordinating defense mechanisms. Emphasis will be given on the neuroendocrine circadian clock cell cycle interactions. We will discuss how the intertwined relations and dynamic characteristics of each component interact with each other and how environmental and pharmacologic interventions alter these relations providing relative advantages and disadvantages to the host in response to external stress. The emerging dynamics plays a central role in the ways hosts mount, compromise or enhance defense responses during interactions with pathogens.
Ioannis (Yannis) AndroulakisIn this talk I will discuss key ideas emphasizing the central role circadian dynamics, and their misalignment, play in orchestrating and coordinating defense mechanisms. Emphasis will be given on the neuroendocrine circadian clock cell cycle interactions. We will discuss how the intertwined relations and dynamic characteristics of each component interact with each other and how environmental and pharmacologic interventions alter these relations providing relative advantages and disadvantages to the host in response to external stress. The emerging dynamics plays a central role in the ways hosts mount, compromise or enhance defense responses during interactions with pathogens. -
 Elissa Schwartz
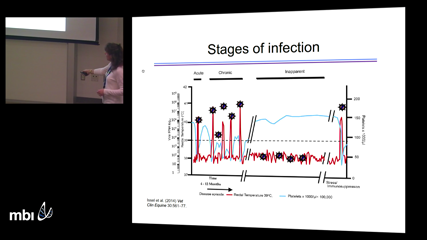
Elissa SchwartzUnderstanding the dynamics of acute viral infection is crucial for developing strategies to prevent and control infection. In this study, lentiviral dynamics in a host without adaptive immunity were examined in order to determine kinetic parameters of infection and quantify the effect of neutralizing antibodies in preventing infection, using mathematical modeling of data from equine infectious anemia virus (EIAV) infection of horses with severe combined immunodeficiency (SCID). Estimated parameters were used to calculate the basic reproductive number and virus doubling time and found that the rate that antibodies neutralized virus was ~18 times greater than the virus clearance rate. These results establish EIAV replication kinetics in SCID horses and the minimal efficacy of antibodies that blocked infection. Furthermore, they indicate that EIAV is at most mildly cytopathic. This study advances our understanding of EIAV infection and may have important implications for the control of other viral infections, including HIV.
-
 Alison Hill
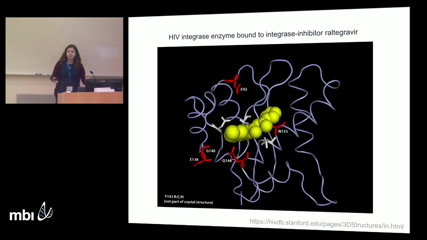
Alison HillHIV can be effectively treated and prevented with antiretroviral therapy, but the evolution of drug resistance can cause treatment failure. Antiviral drugs typically target a specific phase of the virus's life cycle, and it is generally assumed that resistance arises from mutations that alter the virus's susceptibility to the direct action of the drug. Here we consider the alternative possibility that a virus population can evolve towards synchronizing its life cycle with the pattern of drug therapy. The periodicity of the drug treatment could then allow for a virus strain whose life cycle length is a multiple of the dosing interval to replicate only when the concentration of the drug is lowest. This process, referred to as ``cryptic resistance'', could allow the virus population to maximize its overall fitness without having to alter drug binding or complete its lifecycle in the drug's presence. We use mathematical models and stochastic simulations to show that life cycle synchronization can indeed be a mechanism of cryptic viral drug resistance. We show this effect is more likely to occur when the variability in both viral life cycle and drug dose timing are low. More generally, we find that in the presence of periodic drug levels, time-averaged calculations of viral fitness do not accurately predict drug levels needed to eradicate infection, even if there is no synchronization. We derive an analytical expression for viral fitness that is sufficient to explain the drug-pattern-dependent survival of strains with any life cycle length. We discuss the implications of these findings for clinically-relevant antiviral strategies for HIV as well as other viruses including hepatitis B and C and influenza.
-
 Thomas Hoefer
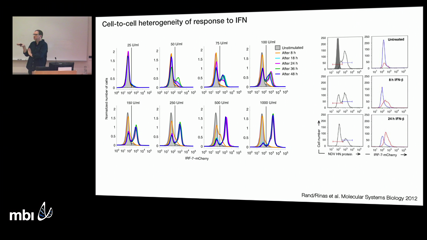
Thomas HoeferThe innate immune system can protect cells from viral infection, suggesting that pathogenic viruses must outpace the innate defense they trigger in order to spread in the host. To study this race, we have developed mathematical models that describe how a susceptible host cell population splits upon virus infection and the ensuing interferon response into infected and protected subpopulations. Confronting these models with time-lapse imaging data of dengue virus infection of lung epithelial cells, we show that the rate at which infected cells transition to productive virus replication controls whether the virus will spread. We corroborate this prediction by quantifying replication dynamics in individual cells. An attenuated dengue virus mutant that is readily recognized by innate immune sensors differs from wildtype virus by having a long transition to productive replication whereas the replication rate itself is hardly affected. These quantitative findings suggest that antiviral drugs that inhibit the formation of replication organelles by dengue virus - and other plus-strand RNA viruses – will synergize with the innate immune response of the host.
[Joint work with Soheil Rastgou, Alessia Ruggieri and Ralf Bartenschlager]
-
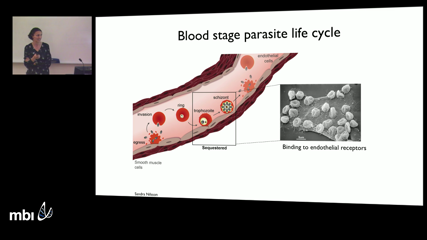 Lauren Childs
Lauren ChildsEach year nearly 200 million people are infected with the malaria parasite, Plasmodium falciparum. One of its most notable features is the variable course and duration of infection experienced by different individuals, ranging from high parasite density, acute and often severe infections to persistent, chronic infections that are often undetectable by microscopy. Field studies examining persistence of infection have used a variety of different genotyping methods, but due to limitations, it is difficult to determine the extent of mixed infections, and nearly impossible to determine if the reemergence of parasitemia is due to a new infection or recrudescence of an existing one. Mathematical models, despite limited knowledge of mechanistic details of host-parasite interactions, have qualitatively reproduced single parasite dynamics observed in patient data. Based on a discrete model of blood-stage parasite dynamics including innate and adaptive immune responses, we analyze simulated output to examine how coinfecting strains, particularly from similar clones that elicit overlapping immune responses, impact infection length and infectiousness. We find that the level of both innate and adaptive immune responses present at the time of coinfection as well as the similarity of the coinfecting strains significantly alters the duration of both the resident and coinfecting strains, particularly during chronic infections. Timing of coinfection also influences the infectivity of the coinfecting strains, likely altering transmission patterns at a population level. Duration of infection and infectivity are critical epidemiological parameters for predicting the efficacy of control strategies, particularly with the looming problem of emerging drug resistance.
-
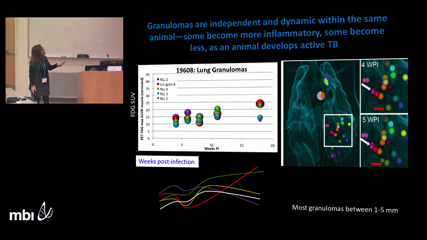 Denise Kirschner
Denise KirschnerWe apply systems biology approaches integrating computational and mathematical modeling with current state of the art data to elaborate the immune response to tuberculosis in the lungs of primates. We use knowledge gained to make predictions about optimal treatment and vaccine protocols.
-
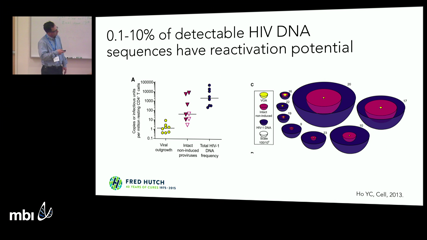 Joshua Schiffer
Joshua SchifferDespite decades of antiretroviral therapy, replication competent HIV persists in roughly 10 million cells. Using several modeling approaches, we demonstrate that cellular proliferation, rather than low levels of viral replication, is the most critical generative mechanism for persistence of infected cells. We then demonstrate that proliferation may serve as the optimal therapeutic target for reservoir reduction, and describe an ongoing clinical trial to test this hypothesis.
-
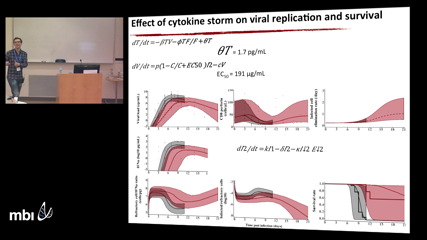 Jeremie Guedj
Jeremie GuedjI will discuss our recent contributions to the field of Ebola virus and the evaluation of favipiravir, a nucleotide analogue initially approved for severe Influenza in Japan. I will show how the techniques of viral dynamics have been used throughout this project, first to find a relevant dosing regimen in humans infected with Ebola virus during the last 2014-2015 outbreak, then to optimize its efficacy using studies in Non-Human Primates. Lastly, I will discuss the use of modeling to better understand the roles of the innate and adaptive immune response in the pathogenesis and the clearance of the infection, as well as the potential application of favipiravir to other viral infections.
