MBI Videos
Workshop 3: Disease Ecology / Eco-epidemiology
-
 Hayriye Gulbudak
Hayriye GulbudakThe emerging threat of a human pandemic caused by high-pathogenic H5N1 avian inuenza virus magnifies the need for controlling the incidence of H5N1 in domestic bird populations. The two most widely used control measures in poultry are culling and vaccination. In this talk, I will discuss mathematical models of avian influenza in poultry which incorporate culling and vaccination. First, we consider an ODE model to understand the dynamics of avian influenza under different culling approaches. Under certain conditions, complex dynamical behavior such as bistability is observed and analyzed. Next, we model vaccination of poultry by formulating a coupled hybrid ODE-PDE model which takes into account vaccine-induced asymptomatic infection. In this study, the model can exhibit the "silent spread" of the disease through asymptomatic infection. We analytically and numerically demonstrate that vaccination can paradoxically increase the total number of infected when the efficacy is not sufficiently high.
-
 Suzanne Robertson
Suzanne RobertsonWest Nile virus (WNV) is a major public health concern in the United States. While seasonal WNV outbreaks have been widely observed to be associated with the end of the avian nesting season, the ecological mechanisms responsible for this synchronicity are poorly understood. Newly hatched birds, or nestlings, have less feather coverage and fewer defense mechanisms than older birds, rendering them more vulnerable to mosquitoes. While total avian population size increases throughout the season, nestling abundance declines at the end of the brooding season. We investigate how this temporal variation in host stage abundance may structure enzootic WNV transmission with a novel mathematical model incorporating avian (host) stage-structure and within-species heterogeneity in the form of stage-specific mosquito (vector) biting rates. We determine the extent to which temporal fluctuations in host stage and vector abundance throughout the season, along with the differential exposure of these stages to mosquito bites, affects the timing and magnitude of WNV activity as well as implications for public health interventions. Specifically, we explore the viability of nestling vaccination as a new form of control in addition to the widely used controls of mosquito larvicide and adulticide.
-
 Xueying Wang
Xueying WangThe transmission of cholera, a water- and food-borne intestinal infection, involves complex interactions among human hosts, pathogens, and the environment. This talk will address the epidemic dynamics of cholera in non-homogenous environments, with a focus on the spatial variation, seasonal fluctuation and bacterial hyperinfectivity, using partial differential equation models.
The presentation will consist of two parts. In the first part, we will discuss seasonality and spatial heterogeneity. The model we employ is built on a reaction-convection-diffusion system to represent the spatial movement of the hosts and pathogens, and incorporates time-periodic parameters to describe the seasonality of the disease transmission and bacterial growth. Using the next generation method, we define and analyze the basic reproduction number of this model, based on which we establish the threshold type results for cholera transmission in a spatiotemporally heterogeneous environment. In the second part, we develop a new modeling framework to study the effect of bacterial hyperinfectivity on cholera epidemics in a spatially non-homogeneous environment. For the second model, the global threshold dynamics is established. The global attractivity of the unique endemic steady state is derived in a special case. We then investigate the dependence of the basic reproduction number on model parameters by theoretical and numerical means.
Our findings highlight the importance of seasonality, hyperinfectivity and their interplay with spatial variation. The result indicates that the prevention and intervention strategies need to take into account the non-homogeneity of the environments in order to effectively control cholera while optimize the use of available resources.
-
 Jorge Velasco-Hernandez
Jorge Velasco-HernandezWe will inevitably face new epidemics where the lack of long time-series data and the uncertainty about the outbreak dynamics make dicult to obtain quantitative predictions. Here we present an algorithm to qualitatively infer time-varying contact rates from short time-series data, letting us predict the start, relative magnitude and decline of epidemic outbreaks. Using real time-series data of measles, dengue, and the current zika outbreak, we demonstrate our algorithm can outperform existing algorithms based on estimating reproductive numbers.
-
 Rebecca Borchering
Rebecca BorcheringAnimals share a variety of common resources, which can be a major driver of conspecific encounter rates. In this work, we implement a spatially explicit mathematical model for resource visitation behaviour in order to examine how changes in resource availability can influence the rate of encounters among consumers. Using simulations and asymptotic analysis, we demonstrate that, under a reasonable set of assumptions, the relationship between resource availability and consumer conspecific encounters is not monotonic. We characterize how the maximum encounter rate and associated critical resource density depend on system parameters like consumer density and the maximum distance from which consumers can detect and respond to resources. The assumptions underlying our theoretical model and analysis are motivated by observations of large aggregations of black-backed jackals at carcasses generated by seasonal outbreaks of anthrax among herbivores in Etosha National Park, Namibia. As non-obligate scavengers, black-backed jackals use carcasses as a supplemental food resource when they are available. While jackals do not appear to acquire disease from ingesting anthrax carcasses, changes in their movement patterns in response to changes in carcass abundance do alter jackals' conspecific encounter rate in ways that may affect the transmission dynamics of other diseases, such as rabies. Our theoretical results provide a method to quantify and analyse the hypothesis that the outbreak of a fatal disease among herbivores can potentially facilitate outbreaks of an entirely different disease among jackals. By analysing carcass visitation data, we find support for our model's prediction that the number of conspecific encounters at resource sites decreases with additional increases in resource availability. Whether or not this site-dependent effect translates to an overall decrease in encounters depends, unexpectedly, on the relationship between the maximum distance of detection and the resource density.
-
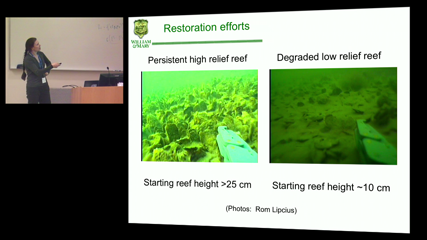 Leah Shaw
Leah ShawUnder the Allee effect, initial populations below a threshold decline, while those above the threshold can persist. First, I will present a model for oyster population dynamics. The model displays bistability, where initially low reefs will degrade but higher ones will persist. Next, I will discuss asymmetric dispersal between two coupled populations under the Allee effect. We explore the bifurcation structure while varying the dispersal rates and threshold. At high Allee thresholds, we find large parameter ranges in which the globally extinct state is the only fixed point, even though uncoupled populations can persist.
-
 Elisabeth Root
Elisabeth RootVery little is known about how spatial distance influences viral subgroup evolution or what local spatio-temporal patterns may look like. This study uses data from a randomized controlled efficacy trial of an 11-valent pneumococcal vaccine (PCV) undertaken in the Bohol province of the Philippines from July 2000 to December 2004. Viral culture and multiplex PCR were done on nasal wash specimens, collected from a sample of infants visiting the regional hospital or outpatient clinics during the vaccine trial. We performed a nested phylogeographic analysis of respiratory syncytial virus (RSV) positive samples and classified virus samples into distinct subgroups. The geographic coordinates of household of residence were obtained for study participants using GPS and used to link phylogenetic results to the geographic location of each patient. We then performed a retrospective space-time scan statistic to identify the spatial location and temporal extent of clusters of each subgroup and visualized geographic patterns using GIS. The spatio-temporal scan statistic identified several unique space-time clusters of RSV-A and RSV-B subgroups. The results show that RSV subgroups arise in distinct localized areas at different points in time, suggesting that spatial distance a population factors play an important role in viral evolution. Spatial analysis and geovisualization is the first step in exploring the effects of distance and potential ecological pressures that contribute to evolutionary pressures.
-
 Linda Allen
Linda AllenHeterogeneity in pathogen transmission is investigated in stochastic multigroup models, with one group representing superspreaders. Superspreaders are characterized as those individuals able to infect a disproportionately high number of susceptible individuals. Recent emerging diseases such as SARS, MERS and Ebola are some examples of outbreaks with superspreading events. We apply continuous-time Markov chains and branching process theory to determine estimates for the probability of a minor or a major epidemic when initiated by a either a superspreader or a non-superspreader. We also examine the time until the outbreak is observed and discuss some applications to emerging and zoonotic infectious diseases.
-
 Benjamin Roche
Benjamin RocheZoonotic pathogens exist within a complex environment that involves many host and other pathogen species. The diversity of host species with low competence for transmitting a given pathogen can reduce the intensity of pathogen transmission, leading to a prophylactic "dilution effect". However, the generality of this effect in wildlife disease systems is unclear, especially because each pathogen can interact with many others across a wide range of host species. Here, we use different theoretical frameworks to examine (i) the expected generality of the dilution effect for vector-borne zoonoses by removing all the assumptions generally involved in dilution effect theory and (ii) how pathogen community can structured when only one host species is present. We finally discuss the different possibilities to combine these two kind of models, in order to identify what is missing to understand if conservation biology can be used as a potential public health tool.
-
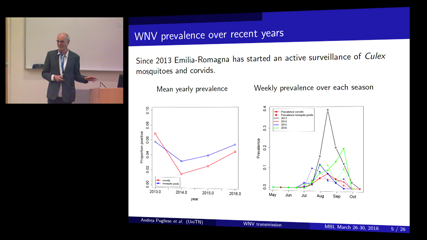 Andrea Pugliese
Andrea PuglieseAn intensified and continuous West Nile Virus spread across Northern Italy has been observed since 2008, which caused more than a hundred reported human infections up until 2016, concentrated in two regions, Emilia-Romagna and Veneto.
We calibrated, using a Bayesian approach, a mathematical model that simulates WNV infection in an avian population with seasonal demography on entomological data collected in Veneto in those years. We compared two assumptions, that the virus is introduced every year at the beginning of the vector breeding season by either infected birds, migrating to the study area, or by diapausing mosquitoes which were infected the previous year. The results suggest that the infection starts every year in infected mosquitoes, supporting the idea that the virus overwinters in the area.
On this basis, we computed seasonal risk curves, indicating the likelihood for a human to be infected, allowing for a temporal shift in vector feeding preferences, according to independent estimates. The highest probability of human infection is estimated for August, consistently with observations.
Finally, multi-year simulations show a qualitative agreement with observed patterns. We are currently working at extending the model to the region Emilia-Romagna (where also data on prevalence in shot corvids are available) and on spatial spread.
Joint work with Giovanni Marini, Roberto Rosà , Marco Tosato, Caterina Rizzo, Gioia Capelli.
-
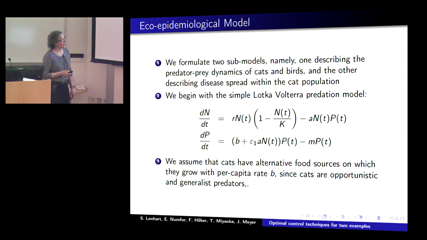 Suzanne Lenhart
Suzanne LenhartTwo examples with optimal control techniques to perform management actions in models with infectious dynamics will be presented. One model with a system of ODEs has predator-prey interactions with disease dynamics in the predator population; one control action increases the level of infection, causing a decrease in the predator population. The second model is a PDE system representing Zika spreading across a state in Brazil; the control varying in space and time is a vaccination rate.
-
 Michael Irvine
Michael IrvineVector biting heterogeneity is believed to be strongly associated with the risk of vector-borne infectious diseases. Understanding the origins of heterogeneity in exposure and risk is important in both control and elimination. Two forms of heterogeneity can characterize the epidemiology of a disease: spatial and individual. These concepts are investigated within the context of lymphatic filariasis (LF), a parasitic, vector-borne disease that has been targeted for elimination.
Infection and mosquito bite data for five villages in Papua New Guinea were used to understand these relationships before and after the introduction of bed-nets. We combine village-based analysis with geospatial modelling to quantify both individual and spatial heterogeneity. The introduction of bed-nets increased biting heterogeneity, but the reduction in mean biting more than compensated for this, by reducing prevalence closer to elimination thresholds. These results are then compared to an individual-based model of LF infection to estimate the impact of the number of years to reach elimination. We find that both spatial and individual heterogeneity are qualitatively different and can have profoundly different policy implications.
-
 Greg Dwyer
Greg DwyerOutbreaks of forest-defoliating insects can have severe impacts on forests, exacerbating climate change, but outbreaks would be even worse if not for epizootics of fatal, species-specific pathogens. An understanding of what determines the timing and severity of pathogen epizootics in forest insects could make it easier to mitigate the effects of outbreaks, but achieving such an understanding is a difficult problem. Work in my lab attempts to solve this problem by using data to choose between competing models of disease dynamics. This approach is common to much of disease ecology, in contrast to many diseases, insect pathogens can be easily used in experiments. It is therefore straightforward, to directly test mechanistic models of disease transmission. By using Bayesian statistical approaches to combine experimental and observational data, we have been able to show that small-scale transmission mechanisms often play a key role in driving large-scale epizootics. This approach has allowed us to disentangle the effects of weather and host density on the spread of a fungal pathogen (Entomophaga maimaiga) of the gypsy moth (Lymantria dispar), which in turn has allowed us to show that the ability of the pathogen to control the insect will likely decline in the future because of global warming. Similarly, we have shown that host heterogeneity in infection risk interacts with host density to determine the severity of epizootics of a baculovirus pathogen of the Douglas-fir tussock moth. We are therefore using our models to guide efforts by the USDA Forest Service to use the baculovirus as an environmentally benign insecticide during tussock moth outbreaks. Combining experimental data with general models of disease spread can thus provide significant assistance to insect pest-control efforts.
