MBI Videos
Workshop 2: Circadian Clocks in Plants and Fungi
-
 David RandI will survey what some recent mathematical results suggest about the design principles behind circadian clocks. In particular, I will discuss flexibility, robustness, buffering mechanisms against environmental heterogeneity, temperature compensation in physiological entrained conditions and tracking of multiple phases. If time permits I will also discuss new methods for fitting reporter time series data to models.
David RandI will survey what some recent mathematical results suggest about the design principles behind circadian clocks. In particular, I will discuss flexibility, robustness, buffering mechanisms against environmental heterogeneity, temperature compensation in physiological entrained conditions and tracking of multiple phases. If time permits I will also discuss new methods for fitting reporter time series data to models. -
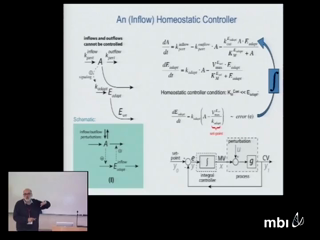 Peter RuoffHomeostatic control mechanisms are essential to keep cells and organisms fit in a changing and challenging environment. An important task is to identify the factors which contribute to the functionality and robustness of homeostatic mechanisms in the presence of environmental perturbations. Kinetic conditions which lead to robust homeostasis and perfect adaptation together with their oscillatory behaviors are described. Analysis of the pathways for nitrate assimilation in plants and Neurospora suggest a homeostatic and oscillatory mechanism for the level of nitrate, apparently to keep the oxidative stress induced by nitrate and nitrate reductase under control during day/night cycles.
Peter RuoffHomeostatic control mechanisms are essential to keep cells and organisms fit in a changing and challenging environment. An important task is to identify the factors which contribute to the functionality and robustness of homeostatic mechanisms in the presence of environmental perturbations. Kinetic conditions which lead to robust homeostasis and perfect adaptation together with their oscillatory behaviors are described. Analysis of the pathways for nitrate assimilation in plants and Neurospora suggest a homeostatic and oscillatory mechanism for the level of nitrate, apparently to keep the oxidative stress induced by nitrate and nitrate reductase under control during day/night cycles. -
 Chris HongIn most eukaryotic organisms, networks of cell cycle and circadian rhythms coexist and work coordinately to create optimal conditions for cells to grow and adapt to the surrounding environment. Cell cycle regulatory mechanisms include multiple checkpoints for controlled growth and cell divisions. The period of this oscillation, however, varies with external conditions such as nutrient and temperature. The cell cycle machinery is optimized for growth and division, but not for time keeping. Circadian rhythms keep track of time and provide temporal regulations in most eukaryotic organisms with a period of about 24 h. In contrast to the period of the cell cycle, the period of circadian rhythms is relatively insensitive to external conditions such as nutrient and temperature. Cell cycle and circadian rhythms are coupled despite of their apparent disparate functions. The circadian gated cell division cycles are observed in various organisms from cyanobacteria to mammals. However, the implications of this coupling on the physiology of an organism are unknown. We use a mathematical model to study interactions between the cell cycle and the circadian clock and their implications in cell cycle regulations.
Chris HongIn most eukaryotic organisms, networks of cell cycle and circadian rhythms coexist and work coordinately to create optimal conditions for cells to grow and adapt to the surrounding environment. Cell cycle regulatory mechanisms include multiple checkpoints for controlled growth and cell divisions. The period of this oscillation, however, varies with external conditions such as nutrient and temperature. The cell cycle machinery is optimized for growth and division, but not for time keeping. Circadian rhythms keep track of time and provide temporal regulations in most eukaryotic organisms with a period of about 24 h. In contrast to the period of the cell cycle, the period of circadian rhythms is relatively insensitive to external conditions such as nutrient and temperature. Cell cycle and circadian rhythms are coupled despite of their apparent disparate functions. The circadian gated cell division cycles are observed in various organisms from cyanobacteria to mammals. However, the implications of this coupling on the physiology of an organism are unknown. We use a mathematical model to study interactions between the cell cycle and the circadian clock and their implications in cell cycle regulations. -
 Deborah Bell-PedersenAbout 20% of Neurospora genes are under control of the circadian clock system at the level of transcript accumulation, and the bulk of the clock-controlled mRNAs have peak accumulation in the late night to early morning. These data suggested the existence of global mechanisms for rhythmic control of gene expression. Consistent with this idea, we found that the Neurospora OS pathway, a phosphorelay signal transduction pathway that responds to changes in osmotic stress, functions as an output pathway from the FRQ/WCC. ChIP/Solexa sequencing with known oscillator proteins revealed that phosophorelay/MAPK pathway components are direct targets of the White Colar Complex (WCC), providing a direct connection between the clock and the output pathway. Activation of the OS pathway by the FRQ/WCC oscillator culminates in rhythmic OS-2 MAPK activity, which through time-of-day-specific activation of downstream effector molecules, controls rhythms in several target clock-controlled genes. Hijacking conserved signaling pathways by the circadian clock provides a new paradigm for global rhythmic control of target genes of the pathway.
Deborah Bell-PedersenAbout 20% of Neurospora genes are under control of the circadian clock system at the level of transcript accumulation, and the bulk of the clock-controlled mRNAs have peak accumulation in the late night to early morning. These data suggested the existence of global mechanisms for rhythmic control of gene expression. Consistent with this idea, we found that the Neurospora OS pathway, a phosphorelay signal transduction pathway that responds to changes in osmotic stress, functions as an output pathway from the FRQ/WCC. ChIP/Solexa sequencing with known oscillator proteins revealed that phosophorelay/MAPK pathway components are direct targets of the White Colar Complex (WCC), providing a direct connection between the clock and the output pathway. Activation of the OS pathway by the FRQ/WCC oscillator culminates in rhythmic OS-2 MAPK activity, which through time-of-day-specific activation of downstream effector molecules, controls rhythms in several target clock-controlled genes. Hijacking conserved signaling pathways by the circadian clock provides a new paradigm for global rhythmic control of target genes of the pathway. -
 Woody HastingsSeveral different circadian rhythms, as well as an annual rhythm, have been studied in the marine dinoflagellate Gonyaulax polyedra (now Lingulodinium polyedrum), many features of which may be grist for modeling mills, whatever they may be. The rhythm of bioluminescence provides an easy "hand" for the automation of its measurement in vivo, and the luciferase and luciferin responsible serve as unambiguous biochemical correlates. This presentation will present highlights of several of its features, starting the subject of the title: post transcriptional control of circadian protein expression. The term "temperature compensation" was introduced in this system to replace "temperature independence" used earlier, based on the observation that the rhythm has a Q10 less than 1. Rhythmicity is lost at low temperatures and bright light (also observed in other systems) but reverts simply upon the return to permissive conditions, with the phase always determined by the time at which permissive conditions were restored. As first reported in humans, different rhythms may have different periods under some conditions - still not well defined in any system. And different rhythms have characteristically different phase angle relationships, but these can be altered by conditions. Might circadian phase be affected by a humoral factor(s)? This is of continuing interest in the mammalian SCN, where dozens of peptides have recently been reported; a unicell might be a favorable system to investigate the possibility. And do models for circadian systems have applicability to infra- ultradian rhythms? A circannual rhythm in Gonyaulax may be a good challenge for elucidating mechanisms. These and other aspects of circadian rhythms in Gonyaulax will be presented for discussion.
Woody HastingsSeveral different circadian rhythms, as well as an annual rhythm, have been studied in the marine dinoflagellate Gonyaulax polyedra (now Lingulodinium polyedrum), many features of which may be grist for modeling mills, whatever they may be. The rhythm of bioluminescence provides an easy "hand" for the automation of its measurement in vivo, and the luciferase and luciferin responsible serve as unambiguous biochemical correlates. This presentation will present highlights of several of its features, starting the subject of the title: post transcriptional control of circadian protein expression. The term "temperature compensation" was introduced in this system to replace "temperature independence" used earlier, based on the observation that the rhythm has a Q10 less than 1. Rhythmicity is lost at low temperatures and bright light (also observed in other systems) but reverts simply upon the return to permissive conditions, with the phase always determined by the time at which permissive conditions were restored. As first reported in humans, different rhythms may have different periods under some conditions - still not well defined in any system. And different rhythms have characteristically different phase angle relationships, but these can be altered by conditions. Might circadian phase be affected by a humoral factor(s)? This is of continuing interest in the mammalian SCN, where dozens of peptides have recently been reported; a unicell might be a favorable system to investigate the possibility. And do models for circadian systems have applicability to infra- ultradian rhythms? A circannual rhythm in Gonyaulax may be a good challenge for elucidating mechanisms. These and other aspects of circadian rhythms in Gonyaulax will be presented for discussion. -
 Qiong YangCyclic processes in biology span a wide dynamic range, from the sub-second periods of neural spike trains to annual rhythms in animal and plant reproduction. Even an individual cell exposed to a constant environment may exhibit many parallel periodic activities with different frequencies. It is therefore important to elucidate how multiple clocks coordinate their oscillations. Circadian oscillation and cell cycle are the two most essential rhythmic events present in almost all organisms. In several unicellular organisms and higher vertebrates, it has been shown that the circadian system affects whether cell division is permitted. Here, we integrate theoretical and experimental approaches to investigate how the circadian and cell division subsystems are coupled together in single cells of the cyanobacterium Synechococcus elongatus. We simultaneously tracked cell division events and circadian phases of individual cells. We found that the timing of cell divisions is synchronized to circadian signals rather than uniformly distributed throughout the day as expected from un-coupled clocks. This suggests that the circadian clock acts as a 'gate' for cell divisions. We fit the data to a model to determine the gating function that describes when cell cycle progression slows as a function of circadian and cell cycle phases. We infer that cell cycle progression in cyanobacteria slows during a specific circadian interval but is uniform across cell cycle phases. Our model is applicable to the quantification of the coupling between biological oscillators in other organisms.
Qiong YangCyclic processes in biology span a wide dynamic range, from the sub-second periods of neural spike trains to annual rhythms in animal and plant reproduction. Even an individual cell exposed to a constant environment may exhibit many parallel periodic activities with different frequencies. It is therefore important to elucidate how multiple clocks coordinate their oscillations. Circadian oscillation and cell cycle are the two most essential rhythmic events present in almost all organisms. In several unicellular organisms and higher vertebrates, it has been shown that the circadian system affects whether cell division is permitted. Here, we integrate theoretical and experimental approaches to investigate how the circadian and cell division subsystems are coupled together in single cells of the cyanobacterium Synechococcus elongatus. We simultaneously tracked cell division events and circadian phases of individual cells. We found that the timing of cell divisions is synchronized to circadian signals rather than uniformly distributed throughout the day as expected from un-coupled clocks. This suggests that the circadian clock acts as a 'gate' for cell divisions. We fit the data to a model to determine the gating function that describes when cell cycle progression slows as a function of circadian and cell cycle phases. We infer that cell cycle progression in cyanobacteria slows during a specific circadian interval but is uniform across cell cycle phases. Our model is applicable to the quantification of the coupling between biological oscillators in other organisms.
Work done in Alexander van Oudenaarden laboratory at MIT and in collaboration with Susan Golden at UCSD.
1. Q. Yang*, B. F. Pando*, G. Dong, S. S. Golden, and A. van Oudenaarden. Circadian gating of the cell cycle revealed in single cyanobacterial cells. Science 327, 1522 (2010).
2. G. Dong, Q. Yang, Q. Wang, Y. Kim, T. L. Wood, K. W. Osteryoung, A. van Oudenaarden, and S. S. Golden. Elevated ATPase activity of KaiC applies a circadian checkpoint on cell division in Synechococcus elongatus. Cell 140, 529 (2010). -
 Yi LiuNo description available.
Yi LiuNo description available. -
 Gisele OdaSeveral experimental studies have altered the natural phase relationship between photic and non-photic zeitgebers, in order to assess their hierarchy in the entrainment of circadian rhythms. In order to interpret the complex results that emerge from these conflicting zeitgeber protocols, we present computer simulations of two coupled oscillator systems forced by two independent zeitgebers. First proposed in 1959 by Pittendrigh and Bruce to model results of their studies on the light and temperature entrainment of eclosion in Drosophila, such a circadian system is also coherent with recent data from many organisms. Our simulations show how the phase of a circadian rhythm varies with a systematic change of the phase relationship between two zeitgebers. "Phase-jumps" and hysteresis in the overt rhythm are shown to arise if the inter-oscillator coupling is high in relation to the zeitgeber strength. Changes in the structural symmetry of the system indicated that these results are expected for a wide range of system configurations, while reproduction of the same phenomena with a simpler model, considering phase effects only, added to the generality of conclusions. We argue that our modeling approach can serve as a conceptual framework for understanding, planning and interpreting conflicting zeitgeber experiments.
Gisele OdaSeveral experimental studies have altered the natural phase relationship between photic and non-photic zeitgebers, in order to assess their hierarchy in the entrainment of circadian rhythms. In order to interpret the complex results that emerge from these conflicting zeitgeber protocols, we present computer simulations of two coupled oscillator systems forced by two independent zeitgebers. First proposed in 1959 by Pittendrigh and Bruce to model results of their studies on the light and temperature entrainment of eclosion in Drosophila, such a circadian system is also coherent with recent data from many organisms. Our simulations show how the phase of a circadian rhythm varies with a systematic change of the phase relationship between two zeitgebers. "Phase-jumps" and hysteresis in the overt rhythm are shown to arise if the inter-oscillator coupling is high in relation to the zeitgeber strength. Changes in the structural symmetry of the system indicated that these results are expected for a wide range of system configurations, while reproduction of the same phenomena with a simpler model, considering phase effects only, added to the generality of conclusions. We argue that our modeling approach can serve as a conceptual framework for understanding, planning and interpreting conflicting zeitgeber experiments.
Work done in collaboration with Daniel S.C.Damineli and Andreas Bohn (Universidade Nova de Lisboa) and W. Otto Friesen (University of Virginia). -
 Didier Gonze, Didier Gonze
Didier Gonze, Didier GonzeCircadian rhythms represent one of the more conspicuous examples of biological rhythms. Manifested at the physiological, behavioral, and cellular levels, these 24-hour rhythms originate at the molecular level, through a complex gene regulatory network. In mammals, the circadian pacemaker is located in the suprachiasmatic nuclei of the hypothalamus (SCN). We have developed deterministic models using non-linear ordinary differential equations that account for the occurrence of autonomous circadian oscillations in single cells, for their entrainment by light-dark cycles, and for their phase shifting by light pulses. The model can be used to unravel the links between molecular alterations (e.g. mutations in clock genes) and clock-related physiological pathologies (such as sleep phase disorders). We have investigated the coupling between the SCN cells and proposed a synchronization mechanism based on periodic neurotransmitter release. Numerical analysis of the model predicts that (1) efficient synchronization is achieved when the average neurotransmitter concentration dampens individual oscillators and (2) phases of individual cells are governed by their intrinsic periods. These results illustrate the possible interplay between the single-cell oscillator and the inter-cellular coupling mechanisms.
-
 Tanya LeiseNo description available.
Tanya LeiseNo description available. -
 Stuart BrodyAmplitude is a measurable parameter of an oscillator, yet it is often not considered as a variable, Amplitude can be measured in several ways: 1) as an output of an oscillator; 2) directly as the amplitude of a "key" clock protein; or 3) indirectly via a Phase-response curve. Data will be presented for a particular mutant (frq7) of Neurospora which shows how the amplitude was altered in all three of the measures listed above. A model will be presented based on limit-cycle expansion which accounts for these observations.
Stuart BrodyAmplitude is a measurable parameter of an oscillator, yet it is often not considered as a variable, Amplitude can be measured in several ways: 1) as an output of an oscillator; 2) directly as the amplitude of a "key" clock protein; or 3) indirectly via a Phase-response curve. Data will be presented for a particular mutant (frq7) of Neurospora which shows how the amplitude was altered in all three of the measures listed above. A model will be presented based on limit-cycle expansion which accounts for these observations.
Temperature affects the amplitude of circadian oscillators in almost all systems studied. Data will be presented to illustrate how an increase in temperature leads to an increase in amplitude of these oscillators from many organisms. This increase in amplitude is proposed to be the mechanism of temperature-compensation, ie. to compensate for the increase in rates at a higher temperature, there is an expansion of the limit-cycle. This model is designated as the temperature-amplitude model, or the "T-A" model. A combination of this model with the one mentioned above predicts how much the midpoint of an oscillator will change when the temperature is raised, a feature not found in other models.
To determine if the temperature-amplitude relationship was a general one, a model callled the "degrade and fire" model was explored. This model simulates the known in vivo oscillations of a synthetic circuit, the arabinose circuit, constructed in E. coli. Data will be presented showing how the change in the activation energy of just one reaction can increase the amplitude of this oscillator, and can convert this non- temperature-compensated oscillator into a temperature-compensated oscillator.
Work done in collaboration with Lev Tsimring.