-
Peter Anderson
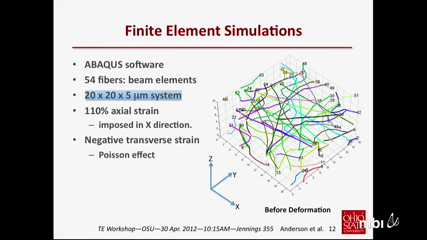
The use of scaffolds for tissue engineering involves aspects of mechanotransduction that are controlled by scaffold properties and structure at the local, cellular scale. For fibrous, electrospun scaffolds, such features include the local fiber stress-strain behavior, fiber density, and undulations in fiber orientation. These serve to provide variations in local stiffness and anisotropy that cannot be quantified through macroscopic testing alone. A combined computational-experimental approach is adopted whereby finite element simulations of electrospun scaffolds are used to link the macroscopic stress-strain response to underlying fiber geometry and fiber stress-strain response. These simulations capture the discrete fiber-straightening, reorientation, and fiber-fiber contact that occurs during scaffold deformation. They can also provide scaffold "Green's functions" to quantify local response to concentrated forces exerted by cells, enabling extraction of "cellular force footprints" in principle. The present simulations are informed by actual fiber geometries from high-resolution confocal microscopy images and macroscopic stress-strain data. An output is the local fiber stress-strain response, which is notoriously difficult to obtain by direct experimental measurement. The calibrated simulations underscore the highly non-uniform (non-affine) and anisotropic nature of the deformation. They also reveal the scale-dependent nature of mechanical response. The talk concludes with challenges to simulation "scale-up" and other pertinent issues. This work is supported by a Multidisciplinary Team Grant, Institute for Materials Research, The Ohio State University.
-

Robert Diegelman
The normal healing response begins the moment the tissue is injured. As the blood components spill into the site of injury, the platelets come into contact with exposed collagen and other elements of the extracellular matrix. This contact triggers the platelets to release clotting factors as well as essential growth factors and cytokines such as platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-ß). Following hemostasis, the neutrophils then enter the wound site and begin the critical task of phagocytosis to remove foreign materials, bacteria and damaged tissue. As part of this inflammatory phase, the macrophages appear and continue the process of phagocytosis as well as releasing more PDGF and TGFß. Once the wound site is cleaned out, fibroblasts migrate in to begin the proliferative phase and deposit new extracellular matrix. The new collagen matrix then becomes cross-linked and organized during the final remodeling phase. In order for this efficient and highly controlled repair process to take place, there are numerous cell-signaling events that are required. In pathologic conditions such as non-healing pressure ulcers, this efficient and orderly process is lost and the ulcers are locked into a state of chronic inflammation characterized by abundant neutrophil infiltration with associated reactive oxygen species and destructive enzymes. Healing proceeds only after the inflammation is controlled. On the opposite end of the spectrum, fibrosis is characterized by excessive matrix deposition, contraction and reduced remodeling. New technologies utilizing PTFE tube implantation have been developed to analyze inflammation and tissue repair in humans. On days 3, 5, 7 and 14 the tubes are removed and the newly deposited cells and matrix components are characterized using histologic, immuno-staining and proteomic analysis. These ongoing studies will be discussed.
-

Praveen Arany
With the renewed excitement in the inducible stem cell field, regenerative medicine is poised at our ability to efficiently direct differentiation of stem cells into functional tissues and organ systems. Besides the vast amount of work currently addressing the mechanistic underpinnings of the directed differentiation process, practical tools to harness this into a clinical utility are lacking. In this talk, I present our work with low power lasers as an innovative tool for clinical regenerative applications. Our current work has uncovered the physical, chemical and molecular events mediating the molecular mechanism mediating these effects using a wide range of in vitro analytical techniques. These observations were validated in vivo assessing directed differentiation of adult dental stem cells in animal models. In summary, low power laser can directs differentiation of resident stem cells via activation of endogenous morphogen.
-
Victor Barocas
Multiscale Models of Collagenous Materials.
-

Edward Sander
Multiscale mechanical interactions are scale spanning physical interactions between the tissue and the extracellular matrix (ECM). They are involved in a variety of biological phenomena, including tissue growth, remodeling, disease, and damage. These interactions are important to characterize because they control both the mechanical behavior of the tissue and the manner in which mechanical signals are propagated to the cellular level. In this talk I will discuss recent work where we incorporate (1) fiber-level rules that govern enzymatic degradation and growth and (2) contractile elements that simulate cell compaction and a redistribution of forces within the surrounding fiber networks into our multiscale modeling framework. Understanding the role of these processes is crucial to comprehending and controlling the integrated response of the mechanical environment in a number of biological contexts.
-

Lauren Black
While the impact of single extracellular matrix (ECM) proteins and mechanical stiffness on cell function have been thoroughly probed individually, little work has been put into to understanding their interactions in the context of cell function. This is particularly important as the ECM is a complex mixture of proteins that change throughout normal development both in composition and stiffness. Recent work by others has demonstrated that cells respond differently to both static substrate stiffness and mechanical stretch when plated on substrates of different compositions. In addition, the effects of growth factor treatment can also be modulated by substrate composition and stiffness. In this talk I will cover our own recent work investigating the effects of alterations in stiffness and composition of the substrate on cardiac differentiation of stem cells and cardiomyocyte proliferation. The system we use is a polyacrylamide gel system with binding sites generated from solubilized decellularized cardiac ECM. This setup effectively allows us to decouple stiffness and composition to investigate their individual roles and any synergistic/ antagonistic effects. Preliminary data indicate that ECM composition and stiffness interact in a complex manner to effect cardiac differentiation of mesenchymal stem cells. Moreover, fetal cardiac ECM composition enhances neonatal cardiomyocyte proliferation over adult cardiac ECM or normal tissue culture plastic.
 Peter AndersonThe use of scaffolds for tissue engineering involves aspects of mechanotransduction that are controlled by scaffold properties and structure at the local, cellular scale. For fibrous, electrospun scaffolds, such features include the local fiber stress-strain behavior, fiber density, and undulations in fiber orientation. These serve to provide variations in local stiffness and anisotropy that cannot be quantified through macroscopic testing alone. A combined computational-experimental approach is adopted whereby finite element simulations of electrospun scaffolds are used to link the macroscopic stress-strain response to underlying fiber geometry and fiber stress-strain response. These simulations capture the discrete fiber-straightening, reorientation, and fiber-fiber contact that occurs during scaffold deformation. They can also provide scaffold "Green's functions" to quantify local response to concentrated forces exerted by cells, enabling extraction of "cellular force footprints" in principle. The present simulations are informed by actual fiber geometries from high-resolution confocal microscopy images and macroscopic stress-strain data. An output is the local fiber stress-strain response, which is notoriously difficult to obtain by direct experimental measurement. The calibrated simulations underscore the highly non-uniform (non-affine) and anisotropic nature of the deformation. They also reveal the scale-dependent nature of mechanical response. The talk concludes with challenges to simulation "scale-up" and other pertinent issues. This work is supported by a Multidisciplinary Team Grant, Institute for Materials Research, The Ohio State University.
Peter AndersonThe use of scaffolds for tissue engineering involves aspects of mechanotransduction that are controlled by scaffold properties and structure at the local, cellular scale. For fibrous, electrospun scaffolds, such features include the local fiber stress-strain behavior, fiber density, and undulations in fiber orientation. These serve to provide variations in local stiffness and anisotropy that cannot be quantified through macroscopic testing alone. A combined computational-experimental approach is adopted whereby finite element simulations of electrospun scaffolds are used to link the macroscopic stress-strain response to underlying fiber geometry and fiber stress-strain response. These simulations capture the discrete fiber-straightening, reorientation, and fiber-fiber contact that occurs during scaffold deformation. They can also provide scaffold "Green's functions" to quantify local response to concentrated forces exerted by cells, enabling extraction of "cellular force footprints" in principle. The present simulations are informed by actual fiber geometries from high-resolution confocal microscopy images and macroscopic stress-strain data. An output is the local fiber stress-strain response, which is notoriously difficult to obtain by direct experimental measurement. The calibrated simulations underscore the highly non-uniform (non-affine) and anisotropic nature of the deformation. They also reveal the scale-dependent nature of mechanical response. The talk concludes with challenges to simulation "scale-up" and other pertinent issues. This work is supported by a Multidisciplinary Team Grant, Institute for Materials Research, The Ohio State University. Robert DiegelmanThe normal healing response begins the moment the tissue is injured. As the blood components spill into the site of injury, the platelets come into contact with exposed collagen and other elements of the extracellular matrix. This contact triggers the platelets to release clotting factors as well as essential growth factors and cytokines such as platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-ß). Following hemostasis, the neutrophils then enter the wound site and begin the critical task of phagocytosis to remove foreign materials, bacteria and damaged tissue. As part of this inflammatory phase, the macrophages appear and continue the process of phagocytosis as well as releasing more PDGF and TGFß. Once the wound site is cleaned out, fibroblasts migrate in to begin the proliferative phase and deposit new extracellular matrix. The new collagen matrix then becomes cross-linked and organized during the final remodeling phase. In order for this efficient and highly controlled repair process to take place, there are numerous cell-signaling events that are required. In pathologic conditions such as non-healing pressure ulcers, this efficient and orderly process is lost and the ulcers are locked into a state of chronic inflammation characterized by abundant neutrophil infiltration with associated reactive oxygen species and destructive enzymes. Healing proceeds only after the inflammation is controlled. On the opposite end of the spectrum, fibrosis is characterized by excessive matrix deposition, contraction and reduced remodeling. New technologies utilizing PTFE tube implantation have been developed to analyze inflammation and tissue repair in humans. On days 3, 5, 7 and 14 the tubes are removed and the newly deposited cells and matrix components are characterized using histologic, immuno-staining and proteomic analysis. These ongoing studies will be discussed.
Robert DiegelmanThe normal healing response begins the moment the tissue is injured. As the blood components spill into the site of injury, the platelets come into contact with exposed collagen and other elements of the extracellular matrix. This contact triggers the platelets to release clotting factors as well as essential growth factors and cytokines such as platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-ß). Following hemostasis, the neutrophils then enter the wound site and begin the critical task of phagocytosis to remove foreign materials, bacteria and damaged tissue. As part of this inflammatory phase, the macrophages appear and continue the process of phagocytosis as well as releasing more PDGF and TGFß. Once the wound site is cleaned out, fibroblasts migrate in to begin the proliferative phase and deposit new extracellular matrix. The new collagen matrix then becomes cross-linked and organized during the final remodeling phase. In order for this efficient and highly controlled repair process to take place, there are numerous cell-signaling events that are required. In pathologic conditions such as non-healing pressure ulcers, this efficient and orderly process is lost and the ulcers are locked into a state of chronic inflammation characterized by abundant neutrophil infiltration with associated reactive oxygen species and destructive enzymes. Healing proceeds only after the inflammation is controlled. On the opposite end of the spectrum, fibrosis is characterized by excessive matrix deposition, contraction and reduced remodeling. New technologies utilizing PTFE tube implantation have been developed to analyze inflammation and tissue repair in humans. On days 3, 5, 7 and 14 the tubes are removed and the newly deposited cells and matrix components are characterized using histologic, immuno-staining and proteomic analysis. These ongoing studies will be discussed. Praveen AranyWith the renewed excitement in the inducible stem cell field, regenerative medicine is poised at our ability to efficiently direct differentiation of stem cells into functional tissues and organ systems. Besides the vast amount of work currently addressing the mechanistic underpinnings of the directed differentiation process, practical tools to harness this into a clinical utility are lacking. In this talk, I present our work with low power lasers as an innovative tool for clinical regenerative applications. Our current work has uncovered the physical, chemical and molecular events mediating the molecular mechanism mediating these effects using a wide range of in vitro analytical techniques. These observations were validated in vivo assessing directed differentiation of adult dental stem cells in animal models. In summary, low power laser can directs differentiation of resident stem cells via activation of endogenous morphogen.
Praveen AranyWith the renewed excitement in the inducible stem cell field, regenerative medicine is poised at our ability to efficiently direct differentiation of stem cells into functional tissues and organ systems. Besides the vast amount of work currently addressing the mechanistic underpinnings of the directed differentiation process, practical tools to harness this into a clinical utility are lacking. In this talk, I present our work with low power lasers as an innovative tool for clinical regenerative applications. Our current work has uncovered the physical, chemical and molecular events mediating the molecular mechanism mediating these effects using a wide range of in vitro analytical techniques. These observations were validated in vivo assessing directed differentiation of adult dental stem cells in animal models. In summary, low power laser can directs differentiation of resident stem cells via activation of endogenous morphogen. Victor BarocasMultiscale Models of Collagenous Materials.
Victor BarocasMultiscale Models of Collagenous Materials. Edward SanderMultiscale mechanical interactions are scale spanning physical interactions between the tissue and the extracellular matrix (ECM). They are involved in a variety of biological phenomena, including tissue growth, remodeling, disease, and damage. These interactions are important to characterize because they control both the mechanical behavior of the tissue and the manner in which mechanical signals are propagated to the cellular level. In this talk I will discuss recent work where we incorporate (1) fiber-level rules that govern enzymatic degradation and growth and (2) contractile elements that simulate cell compaction and a redistribution of forces within the surrounding fiber networks into our multiscale modeling framework. Understanding the role of these processes is crucial to comprehending and controlling the integrated response of the mechanical environment in a number of biological contexts.
Edward SanderMultiscale mechanical interactions are scale spanning physical interactions between the tissue and the extracellular matrix (ECM). They are involved in a variety of biological phenomena, including tissue growth, remodeling, disease, and damage. These interactions are important to characterize because they control both the mechanical behavior of the tissue and the manner in which mechanical signals are propagated to the cellular level. In this talk I will discuss recent work where we incorporate (1) fiber-level rules that govern enzymatic degradation and growth and (2) contractile elements that simulate cell compaction and a redistribution of forces within the surrounding fiber networks into our multiscale modeling framework. Understanding the role of these processes is crucial to comprehending and controlling the integrated response of the mechanical environment in a number of biological contexts. Lauren BlackWhile the impact of single extracellular matrix (ECM) proteins and mechanical stiffness on cell function have been thoroughly probed individually, little work has been put into to understanding their interactions in the context of cell function. This is particularly important as the ECM is a complex mixture of proteins that change throughout normal development both in composition and stiffness. Recent work by others has demonstrated that cells respond differently to both static substrate stiffness and mechanical stretch when plated on substrates of different compositions. In addition, the effects of growth factor treatment can also be modulated by substrate composition and stiffness. In this talk I will cover our own recent work investigating the effects of alterations in stiffness and composition of the substrate on cardiac differentiation of stem cells and cardiomyocyte proliferation. The system we use is a polyacrylamide gel system with binding sites generated from solubilized decellularized cardiac ECM. This setup effectively allows us to decouple stiffness and composition to investigate their individual roles and any synergistic/ antagonistic effects. Preliminary data indicate that ECM composition and stiffness interact in a complex manner to effect cardiac differentiation of mesenchymal stem cells. Moreover, fetal cardiac ECM composition enhances neonatal cardiomyocyte proliferation over adult cardiac ECM or normal tissue culture plastic.
Lauren BlackWhile the impact of single extracellular matrix (ECM) proteins and mechanical stiffness on cell function have been thoroughly probed individually, little work has been put into to understanding their interactions in the context of cell function. This is particularly important as the ECM is a complex mixture of proteins that change throughout normal development both in composition and stiffness. Recent work by others has demonstrated that cells respond differently to both static substrate stiffness and mechanical stretch when plated on substrates of different compositions. In addition, the effects of growth factor treatment can also be modulated by substrate composition and stiffness. In this talk I will cover our own recent work investigating the effects of alterations in stiffness and composition of the substrate on cardiac differentiation of stem cells and cardiomyocyte proliferation. The system we use is a polyacrylamide gel system with binding sites generated from solubilized decellularized cardiac ECM. This setup effectively allows us to decouple stiffness and composition to investigate their individual roles and any synergistic/ antagonistic effects. Preliminary data indicate that ECM composition and stiffness interact in a complex manner to effect cardiac differentiation of mesenchymal stem cells. Moreover, fetal cardiac ECM composition enhances neonatal cardiomyocyte proliferation over adult cardiac ECM or normal tissue culture plastic.