MBI Videos
2013 Workshop for Young Researchers in Mathematical Biology
-
 John Tyson
John TysonProgression through the eukaryotic cell cycle is controlled at a series of checkpoints guarding transitions from one phase of the cycle to the next, e.g., G1-to-S, G2-to-M, metaphase-to-anaphase. These checkpoints ensure that a cell has satisfied certain requirements that are necessary for success of the next phase, e.g., that any DNA damage is repaired before the cell replicates its chromosomes in S phase. These transitions are irreversible: as soon as the conditions of the checkpoint are satisfied, the cell proceeds to the next phase and does not subsequently back up to the immediately preceding phase. The irreversibility of these transitions gives the cell cycle its directionality (G1 → S → G2 → M → G1 ...). The genes and proteins governing these checkpoints have been discovered by molecular geneticists, but the mechanistic basis of irreversibility is still a subject of controversy. Many molecular biologists think that the transitions are irreversible because key proteins are chemically degraded at each transition, but we maintain that irreversibility is a consequence of bistability and hysteresis in the underlying regulatory network. To prove this claim, JJT will describe the mechanism of the G1-S transition in some detail, build and analyze a mathematical model of the mechanism, and compare the implications of the model to experimental facts.
-
 Arthur Sherman
Arthur ShermanInsulin is the master hormone that controls fuel usage by body tissues. After a meal, glucose is plentiful and stimulates insulin secretion, which allows muscle and fat to take up glucose. When blood glucose falls, insulin falls as well and tissues revert to using fat as a fuel. Obesity causes insulin resistance, meaning that more insulin is needed to produce a given amount of glucose uptake. If the number of insulin-secreting pancreatic beta cells, or secretion per cell, increases sufficiently, this excess demand for insulin can be met. If expansion of mass is inadequate, type 2 diabetes, a rise in glucose to levels that are harmful to cells, results. Diabetes leads to cardiovascular disease, blindness, kidney failure and premature death. We update the seminal model of Topp et al (J. Theor. Biol. 2001) for the regulation of beta-cell mass by glucose and present a comprehensive picture of how diabetes develops and may either be avoided or reversed. Although many details of the model are in doubt, we show that any successful model results in a bistable bifurcation structure, with normal and elevated glucose levels separated by a threshold. This simple picture unifies and explains a striking diversity of experimental data, including why prevention is much easier than cure and why bariatric surgery is able to reverse longstanding diabetes within a week.
-
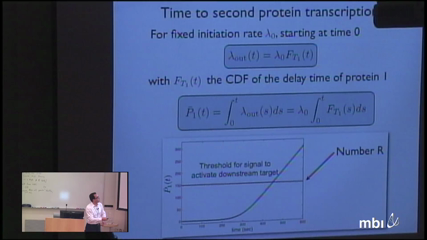 Kresimir Josic
Kresimir JosicSynthetic biology holds the promise of allowing us to engineer living beings. I will start by reviewing some examples where mathematical models lead to the development of synthetic organisms with particular properties: One such example is a synthetic gene oscillator in Escherichia coli that exhibits robust temperature compensation -- it maintains a constant period over a range of ambient temperatures. A mathematical model predicted and experiments confirmed the particular mechanisms that lead to temperature compensation despite Arrhenius scaling of the biochemical reaction rates.
Such successes are encouraging. But how far can our theoretical models take us? I will argue that our models are still fairly coarse, and do not adequately describe all the important properties of genetic signaling networks. For instance, "transcriptional delay" - the delay between the start of protein production and the time a mature protein finds a downstream target - can have a significant impact on the dynamics of gene circuits. Such delay can inhibit transitions between states of bistable genetic networks, as well as destabilize steady states in other networks. I will show how these effects can be described by reduced, non-Markovian models that are quite different from established models. I will also discuss work with experimental collaborators to characterize the distribution of this delay.
-
 Lisa Fauci
Lisa FauciThe process of fertilization in mammalian reproduction provides a rich example of fluid-structure interactions. Spermatozoa encounter complex, non-Newtonian fluid environments as they make their way through the cilia-lined, contracting conduits of the female reproductive tract. The beat form realized by the flagellum varies tremendously along this journey due to mechanics and biochemical signaling. We will present recent progress on integrative computational models of pumping and swimming in both Newtonian and complex fluids that capture elements of this complex dynamical system.
