MBI Videos
Workshop 3: Cancer and the Immune System
-
 Avner Friedman
Avner FriedmanThis is tutorial talk, in which I will introduce the main components of the immune system in the context of cancer. I will introduce the different phenotypes of macrophages, the four classes of CD4+ T cells, and the cytotoxic T cells (CTL) or CD8+ T cell. As will be explained, the tumor may be recognized by macrophages and dendritic cells, and these cells will then activate 'effective' T cells to kill cancer cells. However, the tumor can fight back against the immune system, and in fact it can even use the system to its own advantage, by "educating" macrophages so that they will actually enhance tumor growth by increasing VEGF production. Another factor that works in favor of a tumor are the T regulatory cells, enhanced by the tumor, which inhibit the activities of the effective T cells.
-
 Ami Radunskaya
Ami RadunskayaCancer immunotherapy was announced as the "Breakthrough of the Year 2013" by Science Magazine (December 2013), but there is still a lot of uncertainty in how to design and deliver therapies that boost the immune system’s defense against cancer. In this talk, I will present a brief overview of cancer immunotherapies, including therapeutic cancer vaccines. I will briefly discuss nonspecific and specific immunotherapies, their uses and their drawbacks. I will also highlight how mathematical modeling has been used to understand the effects of immunotherapies, and to design treatment strategies. Most of these models address one of the two main treatment design questions: How Much? and How Often?
-
 Theresa Whiteside
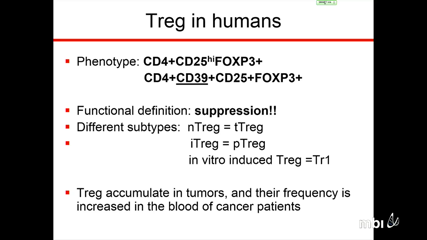
Theresa WhitesideRegulatory T Cells (Tregs) accumulating in the peripheral circulation and tumor sites of patients contribute to tumor escape from the host immune system. Tregs encompass subsets of immune cells with distinct phenotypic and functional properties. Whereas natural (n) or thymic-derived (t) Tregs regulate responses to self-antigens, inducible (i) or peripheral (p) Tregs generated and expanded in regulatory microenvironments control immune responses to a broad variety of antigens.
Human Tregs accumulating in cancer comprise ‘bad’ subsets, which inhibit antitumor immunity, and ‘good’ anti-inflammatory subsets, which maintain tolerance to self and benefit the host. Future therapeutic strategies targeting Tregs will need to discriminate between these Treg subsets and will need to consider reprogramming strategies instead of Treg elimination. Re-establishment of effective antitumor immune responses in cancer patients without disturbing a normal homeostatic T-cell balance will greatly benefit from insights into inhibitory pathways engaged by human tumors.
-
 Ben Wendel
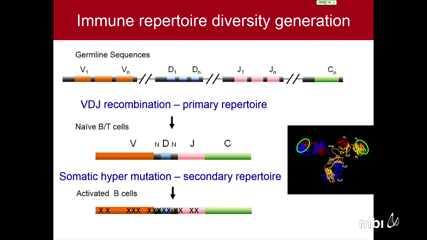
Ben WendelThe immune repertoire has incredible diversity generated through V(D)J recombination to recognize the universe of antigens. This diversity, while crucial for the immune system, makes immune repertoire sequencing (IR-seq) a challenging task. Current sequencing technologies lack the accuracy to delineate between the myriad of minor mutations and PCR/sequencing errors. Using a barcode-driven Molecular IDentifier clustering-based IR-Seq (MIDCIRS), we’ve effectively lowered the error rate for high throughput IR-seq to ~1 in 30,000 nucleotides. With this unprecedented accuracy, we have the power to resolve finer properties of the immune repertoire to characterize responses to disease, treatments, vaccinations, and aging.
On the other end of the spectrum, single cell analysis is critical to elucidate heterogeneity that can be lost in bulk samples. Antigen-specific T cells can be extremely rare, as low as 1 per million T cells, and can have starkly difference phenotypic and functional properties than the bulk. Using a pMHC tetramer-based enrichment strategy, we can isolate the responders to particular diseases and employ our single cell analysis method to simultaneously measure the T cell receptor sequences and gene expression levels. Our single cell analysis technique can be used to investigate the clonal nature and functional capacity of tumor infiltrating lymphocytes – the groups of T cells that are sought to activate by many cancer immunotherapies. This can lead to pretreatment screening and post-treatment disease monitoring methods that can be utilized to provide the optimal treatment regimen for a given patient.
-
 Michael Lotze
Michael LotzeMammalian cells contain hundreds to thousands of mitochondrial DNAs (mtDNA) encoding essential oxidative phosphorylation genes, and can encompass varying percentages of mutant and normal mtDNAs (heteroplasmy) associated with different clinical phenotypes. By generating a set of somatic cell cybrids harboring increasing levels of the pathogenic tRNA 3243A>G mutation [0% mutant (normal), 20-30% (autism & diabetes), 50-90% (neurodegenerative disease), and 100% (Leigh Syndrome)] and assessing changes in mtDNA and nuclear DNA (nDNA) transcriptome by RNA sequencing, Doug Wallace discovered that each clinically relevant mtDNA heteroplasmy level is associated with a unique gene expression profile. Hence, small mitochondrial physiological changes precipitate abrupt changes in cellular signal transduction and epigenomic systems resulting in distinct cellular and clinical phenotypes. Mutations in the 16.6 kilobase human mtDNA can cause a broad spectrum of multi-systemic diseases. Unlike chromosomal genes which are present in only two copies per cell, the mtDNA can be present in hundreds to thousands of copies. If a cell acquires a deleterious mtDNA mutation, this creates an intracellular mixture of mutant and normal mtDNAs, a state known as heteroplasmy. Surprisingly, relatively subtle changes in the heteroplasmic levels can have dramatic effects on a patient’s phenotype. Similarly our group at the University of Pittsburgh has shown profound metabolic changes regulated by the central nuclear protein, HMGB1, evolutionarily ancient and present in all metazoans, driving mitochondrial quality control and serving as a damage associated molecular pattern (DAMP) molecule/target when released for innate and adaptive cell immunity but also promoting autophagy (programmed cell survival) within the cytosol. Nuclear-mitochondrial mismatch can be recognized by innate immune cells but not by adaptive (T and B) cells. Innate immune cells recognize stress ligands on the target cell surface which we hypothesize are promoted in part by important oxidation of critical cysteines in HMGB1.
-
 Sarah Hook
Sarah HookMost cancer therapies are given systemically however we need them to act locally in either lymphoid tissues or in tumors. In this presentation I will discuss some strategies that can be used to turn weapons of mass destruction (or mass immune activation) into precision-guided munitions. Areas where mathematical modeling could aid in the optimal delivery of therapies will also be discussed.
-
 Panel Discussion (Lesinski and Lotze)
Panel Discussion (Lesinski and Lotze) -
 Matthew Farren
Matthew FarrenA major mechanism by which cancers escape control by the immune system is by blocking the differentiation of myeloid cells into dendritic cells (DCs), immunostimulatory cells that activate antitumor T cells. Tumor-dependent activation of signal transducer and activator of transcription 3 (STAT3) signaling in myeloid progenitor cells is thought to cause this block in their differentiation. In addition, a signaling pathway through protein kinase C βII (PKCβII) is essential for the differentiation of myeloid cells into DCs. We found in humans and mice that breast cancer cells substantially decreased the abundance of PKCβII in myeloid progenitor cells through a mechanism involving the enhanced activation of STAT3 signaling by soluble, tumor-derived factors (TDFs). STAT3 bound to previously undescribed negative regulatory elements within the promoter of PRKCB, which encodes PKCβII. We also found a previously undescribed counter-regulatory mechanism through which the activity of PKCβII inhibited tumor-dependent STAT3 signaling by decreasing the abundance of cell surface receptors, such as cytokine and growth factor receptors, that are activated by TDFs. Together, these data suggest that a previously unrecognized cross-talk mechanism between the STAT3 and PKCβII signaling pathways provides the molecular basis for the tumor-induced blockade in the differentiation of myeloid cells, and suggest that enhancing PKCβII activity may be a therapeutic strategy to alleviate cancer-mediated suppression of the immune system.
-
 Yoram Louzoun
Yoram LouzounMany tumors target the specific immune system cells raised to protect the host against tumor. This targeting can take two main shapes. Either the tumor cells attract immune cells to the tumor, enhancing the tumor growth, or they prevent the immune cells from killing existing tumor cells. In both cases a positive feedback loop emerges between the tumor and immune system cell concentrations.
Such a feedback loop may explain the equilibrium obtained between the host immune system and tumors, where tumors stop growing, or grow very slowly, but are not destroyed by the immune response. While tumor size can increase by a factor of 10 within a day, in most cases, this huge division rate is not obtained and in reality the tumor is almost in equilibrium. This equilibrium can be explained by a bi-stable solution of the tumor-immune system dynamics.
We here study a generic set of feedback loops between the immune system cells and their targets in tumors and show that a bi-stable solution can emerge. This solution can occur only if the tumor induces death or inactivation of macrophages. In such a case, a simple negative effect of the pathogens on the macrophages will suffice to induce bistability. The initial conditions then becomes crucial for the solution, given that, according to it, the solution tends to one or the other fixed point, which correspond to a healthy or a sick state.
We show that double inhibition positive feedback loops (immune system kills tumor, which in turn kill immune system cells) behave differently than double activation feedback loops (Tumor produce cytokines that attract immune cells, which in turn produce cytokines which induce tumor growth).Double inhibition feedback loops induce bi-stability in most parameter space, while double activation feedback loops induce such a bistability in very limited regions of parameter space.
-
 Lisette de Pillis
Lisette de PillisWe will present a variety of mathematical models of tumor-immune interactions that have resulted from interdisciplinary collaborations with practicing oncologists and experimentalists. We will discuss certain approaches to modeling cancer growth and immune system interactions, and treatment approaches that harness the power of the immune system to slow and sometimes stop cancer progression.
-
 Mark Robertson Tessi
Mark Robertson TessiT-cell populations are subject to homeostatic control from cytokines and microenvironmental signaling. Disruption of homeostasis can cause changes to the dynamics of the system that have implications for the progression of cancer. Here we present two mathematical models that examine the progression of tumors in the context of T-cell homeostasis. Model 1: During a chronic disease such as cancer, T cells often become tolerant to the antigens presented by the disease. This tolerant state effectively limits the response of the immune system to the tumor. Experimental evidence has shown that depletion of T-cells can lead to a loss of T-cell tolerance. During the homeostatic phase of T-cell compartment repopulation, there is a temporary window of opportunity during which T cells lose their tolerant state, allowing them to respond to tumor antigens. In addition, clonal expansion of the tumor-specific T-cell clone may be enhanced during the regrowth phase due to increased stimulation. We use an ordinary differential equation (ODE) model to explore the effect of T-cell depletion and homeostatic repopulation on the loss of tolerance in the T-cell compartment and subsequent effectiveness of immune-mediated tumor cytotoxicity. The model predicts different outcomes for the tumor and T-cell compartment, dependent on the strength and schedule of the depletion therapy. The optimal regimen can lead to tumor control in some cases, but T-cell exhaustion is also common dynamic predicted by the model. By understanding the effects of T-cell depletion, immune depleting therapies can be optimized to enhance immune potential. Model 2: Large Granular Lymphocytic Leukemia (LGLL) is a T-cell lymphoproliferative disorder that exhibits clonal expansion of a subset of T cells. Since there are no clinical biomarkers to predict the aggressiveness of the disease, treatment decisions are often made on a watch and wait approach. Using a set of ODEs, we develop a model of LGLL that uses clinical patient data from diagnosis to predict the timeframe for progression of the disease. Our experimental results have suggested that the disease is caused by a change in sensitivity to both positive and negative regulators of T-cell homeostasis. The model incorporates these cell-specific mechanisms to investigate their effect when placed in a homeostatic setting. The level of dysregulation as measured from patient-specific data determines the rate of outgrowth of the diseased T-cell clone, and therefore serve as a useful predictive tool for managing treatment decisions in the clinic.
-
 Poster Chalk Talks (5-10 Minutes Each)
Poster Chalk Talks (5-10 Minutes Each) -
 Yang Kuang
Yang KuangA mathematical model of advanced prostate cancer treatment is developed to examine the combined effects of androgen deprivation therapy and immunotherapy. Androgen deprivation therapy has been the primary form of treatment for advanced prostate cancer for the past 50 years. While initially successful, this therapy eventually results in a relapse after two to three years in the form of androgen-independent prostate cancer. Intermittent androgen deprivation therapy attempts to prevent relapse by cycling the patient on and off treatment. Over the past decade, dendritic cell vaccines have been used in clinical studies for the immunotherapy of prostate cancer with some success. Our model examines the efficacy of dendritic cell vaccines when used with continuous or intermittent androgen deprivation therapy schedules. Numerical simulations of the model suggest that immunotherapy can successfully stabilize the disease using both continuous and intermittent androgen deprivation.
