MBI Videos
CTW: Molecular to Systems Physiology
-
 Daniel Cook
Daniel CookHunter and Bassingthwaithe define the Physiome as a set of multiscale, interacting mathematical models of physiology. Although available model repositories are an initial step toward this vision, it is a critical next step to develop computer-readable annotation for connecting codewords across models. Current hand-crafted model-building methods must be formalized and standardized to better support knowledge interaction and sharing. In particular, we argue for semantic annotations as a way of communicating the biophysical meaning of individual model codewords. Once annotated in a computable format, we can automatically find and connect models based on the annotation semantics of the biological entities and physiological properties.
In this talk, we present our approach to semantic annotation, using standard bio-ontology terms to relate physiological properties (e.g. pressure), to anatomical entities (e.g. blood). In turn, we use these annotations to semi-automatically find relevant models from repositories, and ultimately merge those models where appropriate. We present our results with SemGen, a prototype tool, for both building annotations and merging models, even across different modeling languages. If successful, our approach to develop interacting model repositories could accelerate model sharing and integration, and research that depends on the construction of complex models.
-
 Jon Olav Vik
Jon Olav VikVirtual experiments are essential in specifying, assaying, and comparing the behavioural repertoires of computational physiological models. This has applications in model composition, which is crucial for integrative research programmes such as the Virtual Physiological Human and the Human Brain Project. By clearly specifying (sub-) model requirements in terms of expected behaviours under standardised experiments, we envision that model composition could be made much more straightforward, focused and reliable, achieving the industry-level quality management that computational modelling needs to enter the clinical mainstream. A key step is that models and experimental protocols should be represented separately, but annotated so as to facilitate the linking of models to experiments and data. The rigorous, streamlined confrontation between experimental datasets and candidate (sub-) models would enable a "continuous integration" of biological knowledge, in clinical application as well as in model development and basic research.
-
 Kellie Archer
Kellie ArcherOrdinal scales are commonly used to measure health status and disease related outcomes. Notable examples include cancer staging, histopathological classification, adverse event rating, and severity of illness. In addition, repeated measurements are common in clinical practice for tracking and monitoring the progression of complex diseases. Classical likelihood-based ordinal modeling methods have contributed to the analysis of data in which the response categories are ordered and the number of covariates (p) is smaller than the sample size (n). With the emergence of genomic technologies being increasingly applied to identify molecular markers associated with complex disease phenotypes and outcomes, many research studies now include high dimensional feature data where p >> n, so that traditional methods cannot be applied. To fill this void we have developed an innovative penalized random coefficient ordinal response model for classifying and predicting disease progression along with time. Specifically our method extends the Generalized Monotone Incremental Forward Stagewise method (Hastie et al, 2007) to the ordinal response setting in combination with classical mixed effects modeling methods. We demonstrate our method using data from the Inflammation and the Host Response to Injury study in which Affymetrix gene expression profiles and Marshall Multiple Organ Dysfunction Score on six body systems were longitudinally collected at hospitalization day 1 up to day 30 in 169 patients.
-
 Klas Pettersen
Klas PettersenThe baroreflex is a negative feedback system for regulation of blood pressure. Its sensors, the baroreceptors located in the aortic wall and the carotid sinuses, are, however, not pressure sensors, but mechanoreceptors excited by stretch. Here we present a computational physiology model which shows that the increase in arterial stiffness that follows with age is sufficient to account for an overwhelming amount of experimental and clinical data on hypertension. We demonstrate quantitatively that the stiffening causes the baroreceptors to misinform the highly complex machinery responsible for blood pressure regulation. This misinformation occurs because the baroreceptors are strain sensitive, not pressure sensitive, and with stiffening the aortic wall strain ceases to be a good proxy for aortic blood pressure. In contrast to widely held opinions, the results suggest that primary hypertension can be attributed to a mechanogenic etiology without challenging current conceptions of renal and sympathetic nervous system function. And they support the view that a major target for treating chronic hypertension in the elderly is the reestablishment of a proper baroreflex response.
-
 Julia Arciero
Julia ArcieroGlaucoma is the second leading cause of blindness in the world and is characterized by progressive retinal ganglion cell death and irreversible visual field loss. Although elevated intraocular pressure has been identified as the primary risk factor for glaucoma and is the main target of glaucoma treatments, several vascular risk factors that lead to impaired retinal blood flow have also been correlated with the progression and incidence of glaucoma. Here, a multi-scale mathematical model is used to investigate the relative contributions of vascular risk factors on flow regulation and tissue oxygenation in the retina. A previously-developed fluid-structure interaction system modeling the central retinal artery is coupled to a vascular wall mechanics model for the vessels of the retinal microcirculation. Under normal conditions, the model predicts a 14% decrease in retinal perfusion if oxygen demand is decreased by 50% and a 33% increase in perfusion if demand is increased by 50%. These responses are impaired significantly if the metabolic or carbon dioxide mechanisms of retinal blood flow autoregulation are impaired. Changes in oxygen saturation levels in the retinal vascular network are also assessed as levels of mean arterial pressure, oxygen demand, and intraocular pressure are varied. Overall, the model results suggest that impaired autoregulation might increase the risk of retinal ischemic damage, as would occur in glaucoma, under conditions of elevated metabolic demand or decreased mean arterial pressure.
-
 Nicholas Hill
Nicholas HillA novel multiscale mathematical and computational model of the pulmonary circulation is presented and used to analyse both arterial and venous pressure and flow. This work is a major advance over previous studies using structured trees to model vascular beds, e.g. Olufsen et al. (2012), which only considered the arterial circulation. For the first three generations of vessels within the pulmonary circulation, geometry is specified from patient-specific measurements obtained using magnetic resonance imaging (MRI). Blood flow and pressure in the larger arteries and veins are predicted using a nonlinear, cross-sectional-area-averaged system of equations for a Newtonian fluid in an elastic tube. Inflow into the main pulmonary artery is obtained from MRI measurements, while pressure entering the left atrium from the main pulmonary vein is kept constant at the normal mean value of 2 mmHg. Each terminal vessel in the network of `large' arteries is connected to its corresponding terminal vein via a network of vessels representing the vascular bed of smaller arteries and veins. We develop and implement an algorithm to calculate the admittance of each vascular bed, using bifurcating structured trees and recursion. The structured-tree models take into account the geometry and material properties of the `smaller' arteries and veins of radii > 50 microns. We study the effects on flow and pressure associated with three classes of pulmonary hypertension expressed via stiffening of larger and smaller vessels, and vascular rarefaction. The results of simulating these pathological conditions are in agreement with clinical observations, showing that the model has potential for assisting with diagnosis and treatment of circulatory diseases within the lung.
References:
Olufsen, M.S., Hill, N.A., Vaughan, G.D.A., Sainsbury, C. & Johnson, M. (2012) Rarefaction and blood pressure in systemic and pulmonary arteries. J Fluid Mech 705:280-305.
Qureshi, M.U., Vaughan, G.D.A., Sainsbury, C., Johnson, M., Peskin, C.S., Olufsen, M.S. & Hill, N.A. (2014) Numerical simulation of blood flow and pressure drop in the pulmonary arterial and venous circulation, Biomechanics and Modeling in Mechanobiology. ISSN 1617-7959 (doi:10.1007/s10237-014-0563-y )
-
 Frans van de Vosse
Frans van de VosseOne of the main di?culties in the translation of mathematical models to the clinic for supporting clinical decision-making is assessing patient-speci?c values for the model parameters, the boundary and the initial conditions. Measurement modalities or data are not always available for all model parameters. In addition, the precision and accuracy of clinical measurements are hampered by large (biological) variations. Consequently, a balance is needed between the uncertainty resulting from model input parameters and the uncertainty resulting from model assumptions. For this, it is essential to quantify the uncertainty resulting from model input and to determine whether the complexity of the model is su?cient for the application of interest.
The aim of this study is to investigate model personalization (parameter ?xing and prioritization), model output uncertainty, and the number of runs required to reach convergence of their sensitivity estimates (i.e. computational cost) in case of a 1D pulse wave propagation model that was developed to support vascular access surgery planning [1].
The most common and straightforward method is to use crude Monte Carlo simulations in which the model is executed multiple times to estimate the sensitivity indices. This method, however, requires a lot of computational e?ort. Saltelli et al. [2] introduced a method that is computationally less demanding. This makes the method better applicable to computational expensive models or models with many model parameters. However, large computing resources are still required when applying the method to models with many model parameters. Finally, the method of Morris [3] is a global sensitivity analysis that is able to identify the few important model parameters among the many model parameters in the model with a relatively small number of model evaluations.
Our specific aim was to investigate whether model personalization could be performed by ?rst applying the Morris screening method that identi?es the non-important parameters and subsequently applying the Saltelli method to the resulting subset of important parameters. As this is expected to reduce the computational cost of the uncertainty and sensitivity analysis, this might improve clinical applicability. In addition the uncertainty of the model outputs was quantified using the same data that was generated for the sensitivity analysis.
The Saltelli method, which in general requires many model runs, is found to be a robust method for model personalization. Screening for the important parameters using the Morris method is found to work well for the complex cardiovascular wave propagation model for vascular access. The Morris method can therefore be used for parameter ?xing. However, it does not o?er any information in the setting of parameter prioritization, i.e. in identifying which parameters are most rewarding to measure as accurately as possible. The subsets of important parameters identi?ed for the output of interest lead to a significant complexity reduction.
We conclude that for model personalization of complex models it is advised to perform a screening for the important parameters using the method of Morris ?rst, and then perform a variance-based sensitivity analysis on the subset with only important parameters. For this purpose a Saltelli method can be used. Alternative and more computationally e?cient estimation methods not presented in this study are stochastic collocation methods based on polynomial chaos expansion.
[1]W. Huberts, C de Jonge, W.P.M. van der Linden, M.A Inda, J.H.M. Tordoir, F.N. van de Vosse, and E.M.H. Bosboom. A sensitivity analysis of a personalized pulse wave propagation model for arteriovenous ?stula surgery. Part A: Identi?cation of most in?uential model parameters. Med Eng Phys., 35(6):810–26, 2013.
[2]A. Saltelli. Making best use of model evaluations to compute sensitivity indices. Comp Phys Comm, 145:280–297, 2002.
[3]M.D. Morris. Factorial sampling plans for preliminary computational experiments. Technometrics, 33(2):161–174, 1991.
-
 Laura Ellwein
Laura EllweinOne in six adults in the US have some form of coronary artery disease, characterized in particular by accumulation of atherosclerotic plaque. Though stenting is the most common treatment technique, it often leads to restenosis and thrombus formation. Computational modeling of human arteries from patient-specific image-based data offers a noninvasive way to investigate geometry, hemodynamics, and vascular disease corresponding with effects of stenting.
Improved strategies for stent-based patient-specific treatment of atherosclerotic lesions at coronary bifurcations require a greater understanding of normal coronary vessel morphology. We developed a method to quantify morphology in the left coronary artery for eventual use in bifurcating stent design. Computational models of the left main coronary were created from computed tomography (CT) images of 54 patients using ITK-Snap. Metrics assessed using Visualization Toolkit-based software and MATLAB included cross?sectional area, length, eccentricity, taper, curvature, branching law parameters, and bifurcation angles. Traditional statistical analysis using parametric tests for comparing and correlating means revealed significant differences both within and between bifurcations for most metrics.
Image-based computational models for quantifying hemodynamic indices in stented coronary arteries often employ biplane angiography and intravascular ultrasound for 3D reconstruction, but recent advances in optical coherence tomography (OCT) suggest more precise coronary artery reconstruction may be possible. We developed a patient-specific coronary artery reconstruction method that combines OCT, an intravascular imaging modality, with techniques for imaging wire pathway reconstruction adopted from graph theory. The pathway of the imaging wire was determined with a shortest path algorithm assuming minimum bending energy, and OCT images were registered orthogonal to the pathway with appropriate rotational orientation. Segments from both OCT in the stented region and CT upstream and downstream were imported into computational fluid dynamics software to quantify indices of wall shear stress (WSS). WSS results are presented using the method applied to imaging data of a left circumflex coronary artery acquired immediately post-stenting and after a 6-month follow-up period.
Findings from computational modeling studies using patient-specific imaging data may ultimately enhance our knowledge of both healthy coronary arteries and of harmful hemodynamic indices induced by stenting and could be leveraged for future stent design.
-
 Alessandro Veneziani
Alessandro VenezianiWith the progressive inclusion of numerical simulations in medical research and clinical practice, accuracy and reliability of patient-specific computational analyses need to be properly certified. This raises new challenges when estimating patient-specific parameters that may be too difficult or even impossible to measure in practice. On the other hand, these parameters represent a macroscale synthesis
of molecular or mesoscale dynamics, but their practical individual-based quantification based on
modeling arguments is extremely difficult.
Data assimilation techniques are required to merge available data and numerical models to assess the reliability of a quantitative analysis. In this talk, variational procedures will be considered to estimate
(a) vascular compliance from available measures of displacement;
(b) cardiac conductivities from available measures of cardiac potentials.
Some theoretical as well as practical aspects of the numerical solution of these problems will
be addressed.
In particular, we pursue variational techniques based on a constrained minimization approach,
the constraint being represented by the fluid-structure interaction vascular problem
or by the Bidomain equations for electrocardiology.
We will discuss several technical details of this approach.
In general, these techniques lead to high computational costs and proper methods
for the sake of computational efficiency need to be adopted.
We consider in particular both methods based on simplified models for the forward problem
(like the Monodomain equation)
or on surrogate solutions obtained on the basis of the offline/online paradigm, like the Proper Orthogonal Decomposition method (POD). We will illustrate both succesfull experiences as well as pitfalls of these approaches.
-
 Mette Olufsen
Mette OlufsenOrthostatic intolerance occurs when a transition to standing upright causes an imbalance in blood pressure and flow. It affects an estimated 500,000 Americans in particular young women (the female-to-male ratio is approximately 5:1). Symptoms of this disorder range from lightheadedness to fainting. Because many diseases exhibit these symptoms, this disorder can be difficult to diagnose. Moreover, several competing hypotheses have been put forward to explain this disorder, including imbalance of the blood volume regulation and reduced efficacy of the baroreflex control system. The most common tests performed to assess a patient's health are the head-up-tilt and sit-to-stand tests. These tests are designed to stimulate the cardiovascular control system via a simple change of body posture from supine to sitting or standing position. In response to the postural change, blood volume is pooled in the legs leading to a drop in blood pressure in the upper body. The blood pressure drop stimulates baroreceptor neurons, which, via sympathetic stimulation and parasympathetic withdrawal, regulate the heart pumping function and vessel properties facilitating return of blood flow and pressure to their homeostatic levels. This regulation is often disrupted in patients with orthostatic intolerance, often experienced by patients with diabetes, hypertension, and other neurological diseases of which Parkinson’s disease is the most prevalent. The autonomic nervous system is composed of many interacting components, yet measurements done to assess the system are typically limited to heart rate and blood pressure. One way to gain more understanding of the system is via mathematical modeling. This talk will demonstrate what insights can be learned using multiscale models predicting cardiovascular dynamics and the associated autonomic control.
-
 Gheorghe Craciun
Gheorghe CraciunComplex interaction networks are present in all areas of biology, and manifest themselves at very different spatial and temporal scales. Persistence, permanence and global stability are emergent properties of complex networks, and play key roles in the dynamics of living systems.
Mathematically, a dynamical system is called persistent if, for all positive solutions, no variable approaches zero. In addition, for a permanent system, all variables are uniformly bounded. We describe criteria for persistence and permanence of solutions, and for global convergence of solutions to an unique equilibrium, in a manner that is robust with respect to initial conditions and parameter values.
A thorough understanding of these properties will allow for a better understanding of essential biological processes, such as homeostasis and adaptability.
-
 Johnny Ottesen
Johnny OttesenDepression is a widely spread disease: In the Western world approximately 10% of the population experience severe depression at least once in their lifetime and many more experience a mild form of depression. We establish a statistical significant correlation between depression and a recently defined index characterising the hypothalamus-pituitary-adrenal (HPA) axis. The relation supports the common belief that depression is caused by malfunctions in the HPA-axis. We suggest a novel model capable of showing both circadian as well as ultradian oscillations of the hormone concentrations related to the HPA-axis. The fast ultradian rhythm is generated in the hippocampus whereas the slower circadian rhythm is caused by the circadian clock. We show that these patterns fit data from 29 subjects. We demonstrate that patient-specific modelling is capable of making more precise diagnostics and offers a tool for individual treatment plans and more effective design of pharmaceutical molecules as a consequence. Three parameters related to depression are identified by non-linear mixed effects modelling and statistical hypothesis testing. These parameters represent underlying physiological mechanisms controlling the average levels as well as the ultradian frequency and amplitudes of the hormones ACTH and cortisol. The results are promising since they offer an exact aetiology for depression going from molecular level to systems physiology.
-
 Michael Reed
Michael ReedMathematical models of physiological processes allow one to study the homeostatic mechanisms that keep important phenotypic variables within certain normal ranges. When these variables leave the homeostatic range often disease processes ensue. From the models one can derive surfaces that show the relationship between genetic polymorphisms and particularly important phenotypic variables. Known gene polymorphisms correspond to particular points on the surface, some of which are located near the edge of the homeostatic region. The purpose of medical advice tailored to the patient’s genotype is to suggest dietary changes or exercise changes that move the patient back towards the middle of the homeostatic region.
-
 Robert Moss
Robert MossAmong its many functions, the kidney regulates water and sodium excretion, both of which have significant consequences for whole-body homeostasis. A failure to conserve water can lead to death due to dehydration, and a failure to excrete sufficient quantities of sodium can lead to hypertension. To date, mathematical models of renal function have typically treated the kidney as either a "black box", or as a single (homogeneous) nephron. In either case, such models are ill-equipped to predict the consequences of functional changes in the kidney, which may arise in response to neurohumoral regulation, genetic disorders, gene knockouts, the onset of a renal or extra-renal pathology, or the administration of pharmacological interventions. I will discuss our efforts to build a whole-kidney model that explicitly represents the tubular and vascular architecture of the kidney, and which can accurately predict renal water and sodium excretion over a range of physiological conditions.
-
 C. Alberto Figueroa
C. Alberto FigueroaIn this talk we will give an overview of a series of methods for 3D blood flow modeling, ranging from Kalman filtering techniques for automatic outflow and material parameter estimation to baroreflex model for automatic control of blood pressure. We will also discuss recent progress made on the validation of CFD predictions of pressure gradients in coarctation patients at rest and stress using clinical pressure data.
-
 Brian Carlson
Brian CarlsonThe vasculature dynamically responds to a myriad of acute signals reflecting local mechanical conditions, concentrations of neurohumoral substances and metabolic demand in the downstream tissue. The most well known of these mechanisms is the local response of vessels to their intraluminal pressure otherwise know as the myogenic response. Other mechanisms are more global in nature such as the delivery of norepinephrine through sympathetic enervation. In concert with these stimuli we have the conducted response, which is a mechanism acting remotely to convey metabolic state of the downstream tissue to the upstream supply vessels. The common thread of all these regulatory response mechanisms is that the end effectors are the circumferentially oriented vascular smooth muscle cells in the vessel wall that control the dilation and constriction of the vessel.
This talk will present several theoretical models of mechanisms of blood flow regulation some developed at cell level and some at single vessel level resolution, show how these model can be defined from experimental data and then describe how these theoretical models may be utilized in comprehensive models of the cardiovascular system.
-
 Daniela Calvetti
Daniela CalvettiWe present some recent work where how phenomena which occur at the microscopic scale are captured by macroscopic models which lack the fine resolution needed to describe them. This will be illustrated in the context of cellular brain energy metabolism by comparing a spatially distributed model with the capability to account for the proximity of blood vessels and diffusion, and a lumped model which assumes well mixed compartments.
-
 Wen-Hsin Hu
Wen-Hsin HuThere is increasing recognition that sleep-disordered breathing (SDB), which is quite prevalent in obese subjects, can play an independent role in facilitating the development of autonomic and metabolic dysfunction. These abnormalities can lead to the emergence of metabolic syndrome, and subsequently with disease progression, to overt Type 2 diabetes (T2DM). The causal pathways linking SDB to T2DM remain controversial and relatively unexplored. We are developing a large-scale simulation model that would enable competing hypotheses of these causal pathways to be tested at the organ systems level. Our current efforts are based on an integrative model of respiratory, cardiovascular and sleep state control (“PNEUMA�) that was developed by us to characterize the underlying mechanisms that lead to SDB and to determine the effects of SDB on autonomic control of the cardiovascular system and sleep-wake control. We have extended PNEUMA by incorporating a metabolic component, representing the regulation of glucose, insulin, glucagon and free fatty acids using a multi-compartment model. An additional feature is the incorporation of the dynamics of beta-cell regulation. Changes in sympathetic output from the cardiorespiratory portion of PNEUMA, as well as changes in sleep-wake state, lead to changes in epinephrine output and blood flow to the tissues, in turn affecting the metabolism of glucose, insulin and FFA. “Metabolic feedback� takes the form of changes in insulin level, which lead to changes in sympathetic tone through stimulation of the alpha-sympathetic receptors. Consistent with clinical observations, the model predicts that increased severity of sleep apnea, as reflected in an increase in apnea-hypopnea index, leads to higher levels of fasting plasma insulin. Ongoing efforts are aimed at incorporating biological and biochemical processes that occur at the cellular or sub-cellular level, that would enable PNEUMA to simulate disease progression.
-
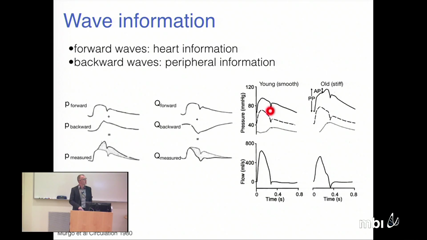 Leif Rune Hellevik
Leif Rune HellevikIn this talk we will present the recent progress in the ongoing development of a framework for the simulation of pressure and flow propagation in arterial networks. Focus will be given on how the effect of uncertainties in model parameters (correlated and uncorrelated) may be quantified. Further, the flexibility of the framework, which allows for the incorporation of organ models (e.g. renal) and multi-scale models for phenotypes such as arterial compliance, will be discussed.
-
 Daniel Beard
Daniel BeardIt is increasingly recognized that multifactorial diseases arise from interaction between genetic and environmental factors, and physiological systems. Examples of particular relevance to human health include the major health burdens that we face: cardiovascular disease and heart failure; metabolic syndrome and type 2 diabetes; and cancer. In all of these examples, acute and chronic (mal)adaptions of specific molecular mechanism and pathways in disease states occur against a background of physiological regulation. Since processes involved in complex disease operate in the context of physiological regulatory mechanisms, an understanding of a disease process builds upon an understanding of the associated physiological systems.
The Virtual Physiological Rat (VPR) is a multi-national research program combining model-driven experiments and experimentally validated multi-scale models to develop theoretical and computational framework explaining: (1.) the long-term regulation of arterial pressure; and (2.) the etiology and sequelae of hypertensive heart disease, spanning molecular genetic to whole-body function. Recent results elucidating novel hypotheses for the mechanisms underlying primary hypertension and the role of metabolic alterations in heart failure will we presented.
-
 Tarun Goswami
Tarun GoswamiBiological systems and their interactions often take place at nanometer level. However, engineering approaches, and modeling biological systems often is at macro or a global level. Examples include devices that mimic the anatomical joints and/or organs replacing them by utilizing the engineering materials in bio environments and follow them up for their durability. During the course of in vivo use of the devices new pathophysiology emerges, affecting other pathways that were not known before. Osteolysis in the case of total joint replacement arises from debris may also be initiated by metal ions. However, engineering approaches are evolving to reduce the debris from the liners in total joint replacements via new manufacturing routes and cross-linking the polymers as well as kinetics of wear rates of the liners. The presentation will show modeling methods utilized to optimize new total joint replacement models of the ankle, and others, understand the tear of anterior cruciate ligament and other injury mechanisms and develop new probabilistic methods to predict the injury occurrences. An overview of device and bone damage mechanics will be presented, at large scale.
-
 Naomi Chesler
Naomi CheslerAccording to Claude Bernard, “the application of mathematics to natural phenomena is the aim of all science, because the expression of the laws of phenomena should always be mathematical.� While much progress has been made in understanding natural phenomena since 1865 when Bernard made this statement and developing mathematical models of these phenomena, much work remains to be done. Whether these models range from the genome to the whole body or are more focused on a particular length-scale, time-scale and organ system, development and validation of physiological, mathematical models still require close collaboration between the theoretician and the experimentalist.
An achievable goal in mathematical modeling today is a model of the cardiovascular system that describes the ejection of blood from the heart, from cross-bridge cycling dynamics to ventricular contraction; incorporates the anatomy, morphometry and biomechanics of the pulmonary and systemic circulations; and is able to connect these systems into one integrated system dependent on and responsible for oxygen delivery, waste removal, and homeostasis. In this presentation, I will share my perspective as an experimentalist. In particular, I will show a set of experimental data that are being used to validate a mathematical model of the heart, pulmonary and systemic circulations and preliminary modeling results. I will also present a vision for more in-depth experimental work that will enable development and validation of a more detailed model with shorter length scales, smaller time scales and better integration between the organ systems with the eventual and lofty goal of the application of mathematics to all cardiovascular phenomena.
-
 John Gennari
John GennariHunter and Bassingthwaithe define the Physiome as a set of multiscale, interacting mathematical models of physiology. Although available model repositories are an initial step toward this vision, it is a critical next step to develop computer-readable annotation for connecting codewords across models. Current hand-crafted model-building methods must be formalized and standardized to better support knowledge interaction and sharing. In particular, we argue for semantic annotations as a way of communicating the biophysical meaning of individual model codewords. Once annotated in a computable format, we can automatically find and connect models based on the annotation semantics of the biological entities and physiological properties.
In this talk, we present our approach to semantic annotation, using standard bio-ontology terms to relate physiological properties (e.g. pressure), to anatomical entities (e.g. blood). In turn, we use these annotations to semi-automatically find relevant models from repositories, and ultimately merge those models where appropriate. We present our results with SemGen, a prototype tool, for both building annotations and merging models, even across different modeling languages. If successful, our approach to develop interacting model repositories could accelerate model sharing and integration, and research that depends on the construction of complex models.
-
 Jefferson Frisbee
Jefferson FrisbeeWith metabolic syndrome (MS) in obese Zucker rats (OZR), the ability of in situ skeletal muscle to resist fatigue is compromised well before muscle function; implicating microvascular or perfusion-based impairments as playing a causal role. However, our results suggest that bulk flow to muscle is not sufficiently constrained to explain the poor performance, and indices such as dilator/constrictor reactivity, vessel wall mechanics and capillary density are not strong predictors of functional outcomes. We have determined that altered RBC distribution at arteriolar bifurcations (?) is increasingly heterogeneous in OZR muscle, iterating to produce a wide heterogeneity of pre-capillary flow distribution vs. controls. This increased spatial heterogeneity of perfusion at bifurcations is not compensated for via temporal switching, rather it is exacerbated owing to blunted temporal activity. The combined effect of these behaviors is that microvascular hematocrit becomes increasingly heterogeneous and fixed, compromising perfusion:demand matching and muscle performance. The magnitude of the deviation of ? from 0.5, and its temporal stability are the strongest predictors of muscle performance to date and reflect a striking loss of system flexibility for microvascular responses to imposed challenges under the setting of elevated cardiovascular disease risk.
