MBI Videos
CTW: Axonal Transport and Neuronal Mechanics
-
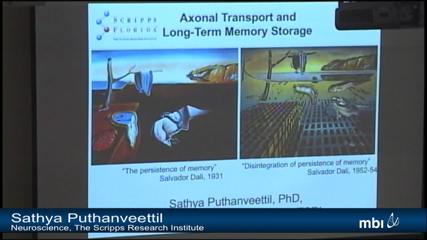 Sathya Puthanveettil
Sathya PuthanveettilLittle is known regarding the identity of the population of proteins and RNAs that are transported to and localized to synapses and how this transport is regulated in neurons. To address this, using the sea slug Aplysia californica and mice, we have begun to study the molecular composition of transport complexes and how the transport is regulated during long-term memory storage. We find that several hundreds of proteins and RNAs are transported by kinesins, the molecular motor that mediate anterograde transport to synapses from the cell body. We further find that axonal transport is regulated in specific neurons for long-term memory storage. These studies bring important insights into the function of axonal transport in synapse formation and memory storage.
-
 Scott McKinley
Scott McKinleyTransport in neurons is intrinsically bidirectional, with each movement modality carried out by molecular motors in either the kinesin (anterograde) or the dynein (retrograde) families. Because all motors are present at a given time there must be competition and/or cooperation among motors that simultaneously bind a single vesicle to nearby microtubules. It has been assumed for much of the last decade that the competition must resolve itself though some kind of tug-of-war; but recent evidence shows conclusively that this is often not the case in vivo. In this talk, we will see a few biological mechanisms (and associated mathematical models) that may lead to resolving theory with experimental observations. Joint work with Will Hancock (Penn State), John Fricks (Penn State), and Pete Kramer (RPI).
-
 Jay Newby
Jay NewbyA key component in the cellular mechanisms underlying learning and memory involves the distribution and delivery of mRNA to synaptic sites in dendrites. A minimal three-state random intermittent search model of motor-driven mRNA transport is developed to explore the question of why motor-driven mRNA are observed moving bidirectionally. The model is analyzed by computing the probability an mRNA is delivered to a synaptic target and the average delivery time (MFPT). It is found that if the branched geometry of the dendrite is ignored, a purely unidirectional transport strategy will result in the smallest MFPT at any given delivery probability. The branched geometry of the dendrite is then incorporated into the model, and it is shown that a phase transition exists for a critical delivery probability where bidirectional strategies improve the corresponding MFPT. To further explore the impact of motor-driven transport behavior on mRNAdelivery, the three-state model is extended to include a detailed, biophysical model of a multimotor complex coordinated through a tug-of-war. The model is analyzed to explore how various measurable, physical quantities, such as adenosine triphosphate, can be tuned to optimize cargo delivery.
-
 David Holcman
David HolcmanNeurite growth is a fundamental process of neuronal development, which requires both membrane expansions by exocytosis and cytoskeletal dynamics. However the specific contribution of these processes has not been yet assessed quantitatively. In this talk, I will present a biophysical model in which we relate the overall neurite outgrowth rate to the vesicle dynamics. We considered the complex motion of vesicles in the cell soma and demonstrated from biophysical consideration, that the main step of finding the neurite initiation site relies mainly on a two dimensional diffusion/sequestration/fusion at the cell surface and we obtain a novel formula for the flux of vesicles at the neurite base. In the absence of microtubules, a nascent neurite initiated by vesicular delivery can only reach a small length. By adding the microtubules dynamics to the secretory pathway and using stochastic analysis and simulations, we showed that the complex dynamics of neurite growth depends on the coupling parameter between the microtubules and the neurite.To validate one aspect of our model, we demonstrated that the experimental flux of TI-VAMP but not Synaptobrevin 2 vesicles contributes to the neurite growth. We conclude that although vesicles can be generated randomly in the cell body, the search for the neurite position using the microtubule network and diffusion is quite fast. Finally our study demonstrates that cytoskeletal dynamics is necessary to generate long protrusion, while vesicular delivery alone can only generate small neurite.
-
 Matthew O'Toole
Matthew O'TooleForces are important for neuronal outgrowth during the initial wiring of the nervous system and following trauma, yet sub-cellular force generation over the microtubule rich region at the rear of the growth cone and along the axon has never been directly measured. Because previous studies have indicated microtubule polymerization and the microtubule associated proteins Kinesin-1 and dynein all generate forces that push microtubules forward, a major question is if the net forces in these regions are contractile or expansive. A challenge in addressing this is that measuring local sub-cellular force generation is difficult. Here we develop the first analytical mathematical model for viscous fluids that describes the relationship between unequal sub-cellular forces arranged in series within the neuron and the net overall tension measured externally. Using force-calibrated towing needles to measure and apply forces, in combination with docked mitochondria to monitor sub-cellular strain, we then directly measure force generation over the rear of the growth cone and along the axon of chick sensory neurons. We find the rear of the growth cone generates 1.99 nN of contractile force, the axon generates 0.64 nN of contractile force and that the net traction force generated by the neuron is 1.27 nN. Together this work suggests that the forward bulk flow of the cytoskeletal framework that occurs during axonal elongation and growth cone pauses occurs because strong contractile forces in the rear of the growth cone pull material forward.
-
 Gianluca Gallo
Gianluca GalloThe formation of a functional nervous system requires the establishment of proper patterns of synaptic connectivity between neurons. Each neuron generates a single axon, but often makes synapses on 100s-1000s of other neurons in disparate parts of the nervous system. The ability of a single axon to generate such complex patterns of connectivity is due to the branching of the axon. Neuronal morphogenesis is dependent on the interactions between the two major components of the cytoskeleton; actin filaments and microtubules. Branches are initiated as actin filament based filopodial protrusions from the main axon shaft, which subsequently mature into branches containing actin filaments and microtubules. This presentation will detail a Monte Carlo simulation of the basic cytoskeletal events underlying the formation of axon branches. The simulation receives empirically derived input values related to aspects of the dynamics of the actin and microtubule cytoskeleton, and returns outputs in the same metric as empirically determined measurement of branch formation. The simulation thus allows direct analysis between empirically derived variables and the final output of the system (i.e., branch formation). The simulation faithfully reproduces the effects of branch inducing factors (e.g., NGF) and suggests new venues of empirical investigation.
-
 Bruce Graham
Bruce GrahamNeurite outgrowth (dendrites and axons) should be a stable, but easily regulated process to enable a neuron to make its appropriate network connections during development. We explore the dynamics of outgrowth in a mathematical continuum model of neurite elongation (McLean & Graham, Proc. R. Soc. Lond. A, 460:2437-2456, 2004; Graham et al, J. Comput. Neurosci., 20:43-60, 2006). The model describes the construction of the internal microtubule cytoskeleton, which results from the production and transport of tubulin dimers and their assembly into microtubules at the growing neurite tip. Tubulin is assumed to be largely synthesised in the cell body from where it is transported by active mechanisms and by diffusion along the neurite. It is argued that this construction process is a fundamental limiting factor in neurite elongation. In the model, elongation is highly stable when tubulin transport is dominated by either active transport or diffusion, but oscillations in length may occur when both active transport and diffusion contribute. Autoregulation of tubulin production can eliminate these oscillations. In all cases a stable steady-state length is reached, provided there is intrinsic decay of tubulin. Small changes in growth parameters, such as the tubulin production rate, can lead to large changes in length. Thus cytoskeleton construction can be both stable and easily regulated, as seems necessary for neurite outgrowth during nervous system development.
In a model variant, we demonstrate competitive growth between two neurite branches being supplied by the same source of tubulin (van Ooyen et al, Neurocomputing, 38-40:73-78, 2001). The faster growing neurite can completely inhibit elongation in the other neurite. Such apparent competition has been observed in real neuron outgrowth.
In a different model formulation, the propensity of neurite branching is assumed to depend on the amount of tubulin reaching the growth cone (Graham & van Ooyen, J. Theor. Biol., 230:421-432, 2004). This transport-limited branching yields matches to the characteristic dendritic morphologies from different neuronal types.
-
 Jeffrey Urbach
Jeffrey UrbachIn the developing nervous system, axons respond to a diverse array of cues to generate the intricate connection network required for proper function. The growth cone integrates information about the local environment and modulates outgrowth and guidance, but relatively little is known about effects of external mechanical or structural cues and internal mechanical forces on growth cone behavior. We have investigated axon outgrowth and force generation on soft elastic substrates for dorsal root ganglion (DRG) neurons (from the peripheral nervous system) and hippocampal neurons (from the central) to see how the mechanics of the microenvironment affect different populations. We find that force generation and stiffness-dependent outgrowth are strongly dependent on cell type. I will discuss recent analyses of the dynamic aspects of growth cone force generation that show surprising regularity underlying the dynamic pattern of traction forces. I will also describe experiments showing that micron-scale confinement affects growth cone shape but, surprisingly, not neurite growth rates. Changes in confinement, by contrast, produce dramatic changes in extension rates. These results suggest a range of opportunities and challenges for developing a quantitative understanding of the influence on engineered environments on axon growth and guidance.
-
 Roberto Bernal
Roberto BernalAbstract Not Submitted
-
 Laura Anne Lowery
Laura Anne LoweryAbstract not submitted.
-
 Timothy Gomez
Timothy GomezGrowth cones interact with the extracellular matrix (ECM) through integrin receptors at adhesion sites termed point contacts. Point contact adhesions link ECM proteins to the actin cytoskeleton through numerous adaptor and signaling proteins. One presumed function of growth cone point contacts is to restrain or “clutch� myosin II-based F-actin retrograde flow (RF) to promote leading edge membrane protrusion. In motile non-neuronal cells, myosin II binds and exerts force upon actin filaments at the leading edge where clutching forces occur. However, in growth cones it is unclear whether similar F-actin clutching forces affect axon outgrowth and guidance. I will show that RF is reduced in rapidly migrating growth cones on laminin (LN) compared to non-integrin binding poly-d-lysine (PDL). Moreover, acute stimulation with LN leads to accelerated axon outgrowth over a time course that correlates with point contact formation and reduced RF. These results suggest that actin RF is restricted by the assembly of point contacts, which we show directly by two color imaging of actin RF and paxillin. Further, using micro-patterns of PDL and LN, we demonstrate that individual growth cones have differential actin RF rates while interacting with two distinct substrata. Opposing effects on actin RF rates were also observed in growth cones treated with chemoattractive and chemorepulsive axon guidance cues known to influence point contact adhesions. Finally, using GFP-actin, we show that actin RF within growth cones in the spinal cord is slow, suggesting RF is being restrained by molecular clutching forces in vivo.
-
 Juergen Reingruber
Juergen ReingruberAxonal transport and growth cone dynamics play a fundamental role for pathfinding and the formation of neuronal networks in the brain. During neuronal development, migrating axons wire the brain by generating long range connections between different brain regions. In the visual system, the retinotopic map connects the retina to the visual centers in the midbrain. When axons from retinal ganglion cells reach the optic tectum (superior colliculus in mammals) they form ordered connections with tectal neurons thereby establishing a topographic map. The map precision is important to correctly transmit the visual information projected onto the retina. Families of ephrin guidance molecule distributed in gradients in the retina and tectum play a crucial role in the map formation process. In addition, engrailed homeoprotein transcription factors are important for axonal guidance. Engrailed displays a graded expression in the chick optic tectum and participates in axonal guidance. Moreover, engrailed regulates the expression of ephrinA5 and increases the sensitivity of growth cones to ephrinA5 repellent activity. The molecular pathway for the engrailed-ephrinA5 interaction involves the internalization of engrailed into the growth cone, which stimulates the production and secretion of ATP, followed by hydrolysis of extracellular ATP into adenosine and the activation of membrane bound
adenosine A1 receptor that are present in higher concentrations in temporal than nasal GCs. Based on these findings, we propose a computational model that shows how the synergetic interaction between engrailed and ephrinA5 increases the precision of the map formation. -
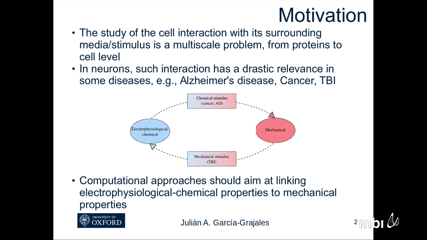 Julian Garcia Grajales
Julian Garcia GrajalesComputational Neuron Mechanics appears as a new multidisciplinary _eld potentially able to study medical challenges such as Alzheimer's disease, tumor growth/migration or traumatic brain injury at cell level. Although computational modeling has been widely used in di_erent Neuroscience applications, the tremendous importance of the interactions between the neuron and its surrounding media/stimulus have been rarely explored. Aimed at analyzing these inter- actions, this work proposes a new multiscale computational framework particularized for two representative scenarios: axonal growth and electrophysiological-mechanical coupling of neu- rites. In the former, the intertwined relation between a biochemical stimulus and the mechanical properties of axons is studied, whereas in the latter, the functional impairments of neurites as a consequence of mechanical constraints are explored. To accomplish these objectives, we de- vise a large scale parallel _nite di_erence program, called Neurite, with the necessary exibility and versatility to implement biological models. In the case of the axonal growth, the program was adapted to simulate microtubule polymerization providing axon mechanical properties as a function of its microtubule occupancy. For the electrophysiological-mechanical coupling case, Neurite was used to relate macroscopic mechanical loading to microscopic strains and strain rates, and to simulate electrical signal propagation along neurites under mechanical loading. For both cases, the models were calibrated against experimental results available in the litera- ture. The growth model showed dramatic variations in the mechanical properties at the tip of the axon, whereas the electrophysiological-mechanical model represents a novelty for predicting the alteration of neuronal electrophysiological function under mechanical loading, thus linking mechanical traumas to subsequent acute functional de_cits.
-
 Bonnie Firestein
Bonnie FiresteinThe precise patterning of dendrites is important for determining how information is processed by a neuron. The neuron cannot receive appropriate information when there is an abnormal decrease in dendrite branching. Thus, disruption of proper signaling networks results. We and others have made significant progress on characterizing extracellular and intrinsic factors that regulate dendrite number or branching by altering cytoskeletal dynamics. Generally, changes to the dendritic arbor are assessed by Sholl analysis or simple dendrite counting. However, we have found that this general method often overlooks local changes to the arbor. We have developed a program (titled "Bonfire") to facilitate digitization of neurite morphology and subsequent Sholl analysis and to assess changes to root, intermediate, and terminal neurites. More recently, we have studied how microtubule dynamics are altered when changes occur to the dendritic arbor. Our future goal is to combine Sholl analysis and microtubule dynamic studies to understand how dendrites assume specific morphologies.
-
 Paul Janmey
Paul JanmeyA common feature of many solid tumors and is that they are stiffer than the normal tissue in which they arise and have increased interstitial fluid pressures and solid tissue stress. These physical changes, which often involve increased synthesis and cross-linking of extracellular matrix protein, can lead to deleterious changes in mechanosensitive cells.Brain and other CNS tissues lack the filamentous protein-based extracellular matrix characteristic of most mesenchymal and epithelial environments, and isolated glioma samples have the same shear storage moduli as normal brain when measured ex vivo at low strains. However, the shear moduli of both normal and malignant brain tissue increase to the kPa range that activates glioma cells and normal glial cells in vitro when the tissue is uniaxially compressed. We suggest that compression stiffening, which might occur with the increased vascularization and interstitial pressure gradients that are characteristic of glioma and other tumors, effectively stiffens the environment of glioma cells and that in situ, the elastic resistance these cells sense might be sufficient to trigger the same responses that are activated in vitro by increased substrate stiffness.
-
 Corina Drapaca
Corina DrapacaIt is well known that mechanical loading and biochemical imbalances can alter brain’s functions and/or structure and lead to neurological diseases. Mathematical models of brain neuro-mechanics can play an important role in highlighting the mechanisms that govern healthy and diseased processes and thus help develop better diagnostic and therapeutic tools. We propose to model the brain as a triphasic composite made of solid, fluid and ionic phases and show some numerical simulations that suggest that normal pressure hydrocephalus could be caused by an imbalance in the salt concentration in the absence of an elevated intracranial pressure. We will also show some numerical simulations of the coupling between the proposed triphasic model and the Hodgkin-Huxley model that permit the investigation of possible linkages between brain’s functionality and mechanical behavior.
