MBI Videos
Workshop 1: Control and Modulation of Neuronal and Motor Systems
-
 Terence SangerAbstract not submitted.
Terence SangerAbstract not submitted. -
 Guillaume Drion
Guillaume DrionNervous system functions are regulated by fast and localized modulation of neural network rhythmic activity. This feature is conserved amongst very different systems, ranging from invertebrate central pattern generators to mammal midbrain and cortical structures. These systems however strongly differ in their structure, function and physiological properties, and are regulated by a large number of interconnected mechanisms, which makes the extraction of key players in the robust regulation of rhythmic activity an arduous task. This talk will introduce a cellular dynamical property called slow regenerativity from which robust and tunable modulation of rhythmic activity can emerge in many different systems, both at the cellular and network levels. It will discuss the ubiquity of slow regenerativity for the control of nervous system activity and illustrate its significance through several key physiological examples.
-
 Leonid Rubchinsky
Leonid RubchinskyDeep brain stimulation (DBS) is used as a therapeutic procedure to treat symptoms of several neurological and neuropsychiatric disorders by controlling electrical activity of neural circuits. In particular it is used to treat motor symptoms of Parkinson’s disease (PD), associated with excessive oscillatory synchronized activity in the beta frequency band. An alternative way to stimulate neural circuits is an emerging technology of optogenetics. It is not clear when/if optogenetics will eventually be possible to implement in clinical practice. However, it is emerging as a widely used experimental tool to control brain networks. Thus, the goal of his study is to explore how effective an optogenetic stimulation in comparison with electrical stimulation in their network effects on elevated synchronized oscillatory activity. We use a computational model of subthalamic and pallidal circuits, which was developed to reproduce experimentally observed beta-band activity patterns. We model electrical stimulation as well as optogenetic stimulation of two types (excitatory via channelrhodopsin and inhibitory via halorodopsin). All three modes of stimulation can decrease beta synchrony. The actions of different stimulation types on the beta activity differ from each other. Electrical DBS and optogenetic excitation have somewhat similar effects on the network. They both cause desynchronization and suppression of the beta-band bursting. As intensity of stimulation is growing, they synchronize the network at higher (non-beta) frequencies in almost tonic dynamics. Optogenetic inhibition effectively reduces spiking and bursting activity of the targeted neurons. We compare the stimulation modes in terms of the minimal effective current delivered to basal ganglia neurons in order to suppress beta activity below a threshold. Optogenetic inhibition usually requires less effective current than electrical DBS to achieve beta suppression. Optogenetic excitation, while as not efficacious as optogenetic inhibition, still usually requires less effective current than electrical DBS to suppress beta activity. Our results suggest that optogenetic stimulation may introduce smaller effective currents than conventional electrical DBS, but still achieve sufficient beta activity suppression. Thus, optogenetic stimulation may be more effective than electrical stimulation in control of synchronized oscillatory neural activity because of the different ways of how stimulations interact with network dynamics.
-
 Amanda Edwards
Amanda EdwardsPeople with damage to their cerebellum often exhibit "reaching ataxia", or misdirected, poorly scaled movement patterns which are reminiscent of a poorly tuned control system. Ataxia affects most all activities of daily living (e.g. eating, cooking, bathing, dressing, working). It is believed that these patients have a static, miscalibrated internal model of their body dynamics; however, it is unknown whether their feedback control is intact. We challenged these participants with visuomotor system identification tasks in order to model their feedback control architecture. Our results suggest that cerebellar patients have intact feedback control, but are forced to rely on time-delayed visual feedback. The key difference is that healthy subjects seem to be able to compensate for their visuomotor delay suggesting that the cerebellum may be serving the role of a Smith predictor for this task. Finally, we were able to leverage the cerebellar patients intact visuomotor feedback control system to improve the scaling of these movements by altering their visual feedback based on their real-time movement in a virtual reality environment.
-
 John Guckenheimer
John GuckenheimerAnimal locomotion results from interactions of rhythmic movements of the body with the environment.
A biomechanical model for the movements may yield an unstable periodic orbit of a dynamical system. In these circumstances, the animal must exercise active control to maintain stability. This lecture will discuss the problem of estimating the Floquet multipliers which characterize local stability properties of a periodic orbit. The information needed depends upon perturbations from the periodic orbit. Animals can either rely upon fluctuations in the environment (''noise'') or generate excitations that move the organism off the orbit to obtain the required information. We discuss these alternatives in terms of the distribution of residuals between the animal's trajectory and the target periodic orbit for its motion.
-
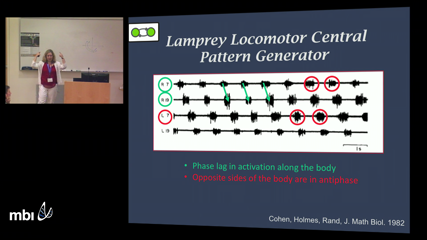 Kathleen Hoffman
Kathleen HoffmanLamprey locomotion involves electrical signal in the central nervous system, to the force generation of the muscle, to the interaction between the body and the environment. Proprioceptive feedback from sensory organs, called edge cells, are known to modulate the rhythm of the electrical signal in the spinal cord. Modeling this set of complex systems involves coupled oscillators, representing the central pattern generator (CPG) for locomotion, a Hill-type muscle model for force generation, and a fluid-elastic body interaction that simulates the body in the fluid environment. Understanding this complex system involves an integrated interdisciplinary approach that combines biological experiments, experimental fluid dynamics, and different types of mathematical models for the CPG, muscle, body and fluids. In this talk, I will discuss the work of an interdisciplinary team of researchers that have combined their expertise to further understand this complex problem.
Joint work with: Eric Tytell (Tufts), Tim Kiemel (UMCP), Lisa Fauci (Tulane), Christina Hamlet (Buchnell), Nicole Massarelli (Schoolcraft College), Megan Leftwich (GWU)
-
 Ivan Soltesz
Ivan SolteszThroughout the cortical mantle, distinct inhibitory cell types deliver GABA to specific spatial domains of principal cells at particular times during behaviorally relevant network oscillations. Recent results from the hippocampus of awake mice have revealed emerging principles of the temporal ordering of interneuronal discharges during network oscillations. We will discuss new evidence showing the highly non-homogeneous organization of inhibitory-excitatory microcircuits in the hippocampus is highly selective with respect to the long-distance projection patterns of the heterogeneous pyramidal cell populations. Next, the question of closed-loop optogenetic control of hippocampal pathological activity will be addressed. Finally, we will discuss results from data-driven, full-scale (1:1) computational models of the hippocampus that give us quantitative insights into the roles of the various constituent cell types in ensemble network activities.
-
 ShiNung Ching
ShiNung ChingUsing stimulation to modulate activity in neuronal circuits has broad applicability in both clinical and basic scientific domains. A pressing need in these applications is the design of principled stimulation inputs that go beyond the ‘square pulse’ type waveforms commonly used in current practice. Such waveforms are likely inefficient and too blunt to achieve neuroscientific goals such as investigations of temporal neural coding. Control theory can help to obviate this issue by optimizing extrinsic inputs for maximizing activity-based objective functions. However, in this regard the complexity and diversity of neural dynamics presents a major analytical challenge for the deployment of classical control methods. Indeed, progress in neurostimulation design and optimization has usually required abstraction of dynamics to low-dimensional canonical models, or assumptions of heterogeneity across a neuronal population. This talk will explore approaches that do not require an explicit mathematical model for the circuit to be controlled. These approaches blend ideas from adaptive control theory, system identification and machine learning, wherein the controller builds a representation of the targeted circuit in an online fashion. The representation is not a biophysical model, but rather a recurrent network construct that approximates the dynamics of the circuit in question. Thus, control can be performed on large, irregularly connected populations of spiking neurons, at the expense of needing sufficient observations to build the representation. Interestingly, for some formulations, the learned control strategy itself produces spiking activity, providing interpretations of how control objectives may manifest intrinsically within neuronal networks.
-
 Cameron McIntyre
Cameron McIntyreAn electrophysiological hallmark of Parkinson’s disease (PD) is abnormal oscillatory activity, most notably excessive synchronization in the beta band (12-20 Hz). These oscillations are seen throughout the cortical-subcortical motor circuitry, and historically have been thought to arise within the basal ganglia (BG). However, recent theoretical and experimental studies have suggested a cortical origin for the abnormal activity. As such, a substantial scientific effort is currently focused on characterizing cortical activity in the parkinsonian state, as well as its modulation by deep brain stimulation (DBS). Hyperdirect neurons have been identified as key players in this puzzle because of their unique anatomical connections and powerful influence over network activity. Hyperdirect neurons are a special subset (~5%) of layer V pyramidal neurons whose corticofugal axon projects down internal capsule and into the spinal cord, while also sending an axon collateral to the subthalamic nucleus (STN). One prevailing hypothesis is that pathological beta activity arises within cortex and is transmitted into the BG via the hyperdirect pathway. In line with this hypothesis, selective optogenetic stimulation of the hyperdirect pathway is sufficient to alleviate parkinsonian symptoms in rodents and numerous recent clinical DBS studies have come to a similar conclusion in humans. My presentation will provide an overview of experimental work analyzing the hyperdirect pathway, followed by new modeling results on DBS of this pathway and its subsequent effect on cortical network activity.
-
 Lena Ting
Lena TingProprioceptive sensory information is essential to movement, particularly in sensorimotor responses to external perturbations to the body. Our data show that rapid increase in resistive force of a passive muscle when stretched, i.e. short-range stiffness may cause enhanced sensory signals that facilitate the detection and predictive response to sudden mechanical perturbations to the body. Importantly, this history-dependent property of muscle spindle firing rates does not have a unique relationship to muscle length or velocity, but rather can be predicted in fine detail based on a unique pseudo-linear transformation between muscle force and the first time derivative of force, dF/dt and muscle spindle afferent firing rate. Several history-dependent features of muscle spindle firing rates can be predicted based on muscle force and dF/dt and are likely due to cross-bridge cycling kinetics in muscle fibers. Such history-dependence is lacking in current models of muscle spindles, but could be necessary to explain a number of phenomena from postural response to perturbation, spasticity, and perception of limb position. Moreover, the encoding of force as a proxy for length in muscle spindles has many implications for normal and impaired control of movement.
-
 Alessio Franci
Alessio FranciFluctuations in neuronal electrical activity span orders of magnitude both in temporal and spatial scales. The fine grain scale of ion channels and single neurons is mathematically captured by nonlinear electrical circuits. The behavior of those circuits is robustly regulated by modulating a few feedback loops that combine the dynamic response of neuronal ionic currents. Positive feedback loops play a key role by creating regions of negative conductance that are responsible for neuronal switching and oscillatory behaviors.
The role of neuronal negative conductance propagates at the medium scale of neuron networks and at the gross scale of whole body circadian rhythms. At the medium scale, it endows networks with rapid and localized switching capabilities that regulate brain information processing (brain states). At the gross scale, it allows robust circadian fluctuations in neuron electrical activity that influence cellular clock oscillations through calcium-dependent signaling pathways, thus closing a feedback loop essential for free-running molecular oscillations.
-
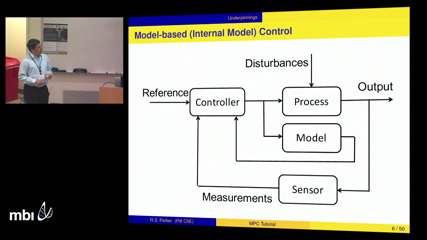 Robert Parker
Robert ParkerThe concept of automated closed-loop feedback control has been used in the chemical industries since the 1950s. While these feedback control methods work well in industrial practice, the challenges of biological systems -- including comparative data sparsity, a lack of full state measurements, challenging dynamics, and nonlinearity -- often lead to undesirable performance using simple feedback controllers. This talk will introduce Model Predictive Control, which serves as the algorithm driving many of the closed-loop insulin pumps in development for insulin-dependent diabetic patients. By developing an accurate model of they physical system, and using that model explicitly in the synthesis and solution of the on-line control problem, performance superior to that offered by feedback control is possible, even for challenging biological systems. This talk will provide an overview of model construction from first-principles knowledge as well as data-driven modeling, as well as the synthesis of the MPC controller to achieve clinically-relevant performance objectives. An example application of these tools will be discussed in the context of glucose control for diabetic and/or critical care patients.
-
 Casey Diekman
Casey DiekmanThe mechanisms underlying oscillations in a central pattern generator (CPG) may differ fundamentally in the intact organism versus an isolated CPG preparation. We investigate this aspect of rhythmogenesis in a computational model of closed-loop respiratory control, incorporating minimal models of the brainstem CPG, lung mechanics, oxygen handling, metabolism, and chemosensation. We analyze the model behavior using fast/slow dynamical systems analysis and open-loop/closed-loop control analysis. We show that eupnea-like bursting oscillations arise from a distinct mechanism in the intact (closed loop) versus isolated (open loop) systems. The closed-loop model exhibits bistability between eupnea-like bursting and tachypnea-like tonic spiking, and we demonstrate that imposed bouts of hypoxia can induce a dramatic transition from eupnea to tachypnea. For moderate bouts of hypoxia, the endogenous properties of the ionic conductances in the Butera-Rinzel-Smith CPG model can lead to spontaneous autoresuscitation and recovery to eupnea. We also find that chemosensory feedback gives the closed-loop control system greater robustness to changes in metabolic demand than the open-loop control system.
-
 Paul Miller
Paul MillerWhen more than one homeostatic process is at work in a given system, there is always a chance the two will compete with each other. For example, if two thermostats in a room control different heating/cooling systems, unless their set-points are identical, one could be causing heating and the other cooling while the temperature lies intermediate between the two set-points. In neurons, multiple homeostatic mechanisms appear to be at play. We focus on two categories, synaptic scaling, which impacts connection strengths between neurons, and intrinsic homeostasis, which impacts the excitability of neurons. We find criteria for the two homeostatic mechanisms to cooperate and produce a stable state of time-averaged neural activity. We show that in these conditions the neuron can respond to stimuli more reliably, because the variance as well as the mean of its firing rate can be controlled with two sensors. Finally, we demonstrate that within a recurrent feedback circuit, a useful computational operation--temporal integration of inputs--can be achieved by these dual control mechanisms operating together, i. e., they robustly achieve the fine-tuning of the circuit that is necessary.
-
 Kenneth Loparo
Kenneth LoparoThis talk will provide an introduction to linear systems theory including (1) dynamical systems terminology and basic concepts (e.g. equilibrium, linearization, stability and control); (2) input/output maps, state space realizations, controllability, observability and stability of linear dynamical systems; (3) estimation and control of linear systems (e.g. observers, linear quadratic regulators, and the separation principle).
-
 Rodolphe Sepulchre
Rodolphe SepulchreFeedback and sensitivity are core concepts of control theory, but the theory is grounded in the frequency-domain analysis of open dynamical systems and mature only for linear time-invariant (LTI) systems. Excitability is a core concept of mathematical physiology, but the theory is grounded in the state-space analysis of closed dynamical systems, with little contact to control theory.
This tutorial will review the three concepts, with the aim of connecting them to address the control and modulation of rhythmic behaviors encountered in biology.
The emphasis will be on experimental questions of neurophysiology. This discipline benefits from a matured modeling framework grounded in circuit theory, a key historical bridge between input-output and state-space modeling.
-
 Andre Longtin
Andre LongtinStochastic optimal control is a technique that allows the determination of a control signal to a noise-driven system to achieve a certain goal while minimizing certain costs. This talk will summarize recent efforts at controlling the firing times of a stochastic excitable system using stochastic optimal control. We consider both the cases where only a knowledge of the previous firing time is available, as well as the more information-rich case where one can monitor the ongoing voltage fluctuations. The talk will also consider the design of stimuli that can best reveal information about system parameters through a combination of information theory and stochastic optimal control. Finally we will present recent work on the control of sensory focus, in which the boundary between tonic and bursting activity serves as an optimal position where the Fisher information about an external input is maximized.
-
 Neville Hogan
Neville HoganDespite vastly slower ‘hardware’ (e.g. muscles) and ‘wetware’ (e.g. neurons) human dexterity and agility significantly out-perform contemporary robots. How is this possible? Slow actuators and long communication delays require predictive control based on some form of internal model—but what form? One possible answer is based on dynamic primitives; they enable highly dynamic behavior with minimal high-level supervision and intervention. Supporting this proposal, I will review a surprising limitation arising from control via dynamic primitives—moving slowly is hard for humans. Controlling physical interaction requires a special class of dynamic primitives, mechanical impedances. Both motion and interaction primitives may be combined by a nonlinear generalization of the classical equivalent electrical circuit. It reconciles contrasting constraints of information-processing (computation) and energy-processing (physical dynamics). I suggest that nonlinear equivalent networks provide a general basis for the internal models required for high-performance interactive control, and especially physical human-robot interaction.
-
 Ansgar Büschges
Ansgar BüschgesThe generation of motor behavior is a main function of all animals’ nervous systems. In the past decades detailed information has become available on the organization and operation of those neuronal networks that generate the motor output needed for locomotion. This encompasses the movement of wings for flying, the trunk and fins for swimming, or legs for walking. Today, a lot is known about the generation of so-called basic or default locomotor patterns, e.g. single leg stepping in walking. In contrast, we are only starting to find out (i) how the networks involved in these behaviors generate flexible motor output allowing for the generation of adaptive locomotor behavior, for instance changes in heading, direction and walking speed, (ii) how these networks interact with each other to generate proper coordination for locomotion, e.g. between the stepping legs of a walking animal, and finally (iii) which aspects of the motor output need to be controlled by the brain and which are under local control in order to allow for the full functional range in locomotion. The talk will present recent findings on these three topics gathered from studies on insect walking, both in the stick insect and the fruit fly.
-
 Dan Wilson
Dan WilsonWhile high-frequency deep brain stimulation (DBS) is a decades old treatment for alleviating the motor symptoms of Parkinson’s disease, its mechanisms are not well understood. Some experimental evidence suggest that DBS works by making neurons fire more regularly, while other seemingly contradictory evidence suggests that DBS works by making neural firing patterns less synchronized. Here, we present theoretical and numerical results that suggest that these two mechanisms are not mutually exclusive. Specifically, in a noisy group of phase oscillators, high frequency perturbations can separate the population into multiple clusters, each with a nearly identical proportion of the overall population. Exploitation of this phenomenon could lead to better DBS protocols.
-
 Dagmar Sternad
Dagmar SternadWhile everyday actions flexibly combine rhythmic and discrete movement elements, motor neuroscience research has largely studied these movement types in isolation, developing separate accounts for these two movement forms. Supported by a series of brain-imaging, behavioral and modeling studies, our premise is that rhythmic and discrete behaviors are two dynamic primitives constituting more complex actions. Our research analyzes how task dynamics constrain and enable actions and their improvement with practice. We start with a mechanical model of the task and render it in a virtual environment with a fully known solution space. Based on mathematical analyses of the modeled task, we study how humans develop solutions to meet complex task demands. Key concepts in our analysis are variability, stability, and predictability. Using three model tasks, throwing a ball, rhythmic bouncing of a ball, and transporting a cup of coffee, we show that humans develop skill by: 1) finding error-tolerant strategies and channeling noise into task-irrelevant dimensions, 2) exploiting solutions with dynamic stability, 3) optimizing predictability of object dynamics. These findings are the basis for developing propositions about the controller: complex actions are generated with dynamic primitives, modules that overcome substantial delays and noise in the neuro-mechanical system. Extending from these experimental platforms we have developed interventions that assess or help restore functional behavior in neurological patients.
-
 Terence Sanger
Terence Sanger
