-

Julia Chifman
Panel discussion and workshop wrap-up led by Julia Chifman
-

Julia Chifman, Julia Chifman
Panel discussion led by Julia Chifman
-

Laura Kubatko
Panel discussion led by Laura Kubatko
-

Kevin Coombes
Panel discussion led by Kevin Coombes
-

Diego Mallo
Panel discussion led by Diego Mallo
-

Marc Suchard
Panel discussion led by Marc Suchard
-
Russell Schwartz
It has long been recognized that cancer is a process of aberrant evolution, in which clonal diversification and selection result in progression from an initially healthy cell through precancerous and successively more aggressive cancerous states. Genomic studies have increasingly elucidated the finer details of these evolutionary processes and how they act in and vary between cancers, but there are still large gaps in our knowledge and our ability to apply it to translational directions. One key gap is our still-limited ability to predict risk of future cancer progression, e.g., which precancerous lesions are likely to progress to cancer, or which early cancers are likely to threaten the patient, to respond or recur following treatment, or to metastasize. Here, we explore the predictive power for progression outcomes of variations patient-to-patient in evolutionary diversification – i.e., risk arising from patient-specific variation in mechanisms of somatic hypermutability – as compared to the predictive power of selection – i.e., risk arising from patient-specific differences in the spectra of driver gene mutations. We estimate these factors using tumor genome sequence data, combining driver mutation calls with quantitative features derived from tumor phylogeny models and overall mutation burdens to assess in a machine learning analysis how these two classes of evolutionary factors collectively predict future cancer progression. Through application to a set of patient cohorts from the Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC), we show that measures of diversification and selection each contribute complementary and partially orthogonal predictive power relative to one another and to conventional clinical predictors of outcome. The work suggests the importance of better characterizing mechanisms of somatic variation in cancers and their role in cancer risk across diverse progression outcomes.
-

Mary Kuhner
Barrett's Esophagus is a neoplastic condition that usually remains stable, but progresses to esophageal adenocarcinoma (EA) in approximately 5% of cases. We used WGS to survey 4 biopsies from 40 EA-outcome and 40 non-EA-outcome Barrett's patients.  These data showed two strongly contrasting patterns. A constellation of genomic-damage indicators including mutations in TP53, copy-number changes, genome doubling, and chromosomal rearrangements were strongly associated with progression to cancer. However, a separate set of features including very high point mutation and indel loads, mutation signature 17, copy-number variation at fragile sites, and positive selection on multiple loci were observed across patients regardless of outcome and showed little to no association with progression. While features in this second group are advantageous in the hostile environment of the Barrett's segment, they are not sufficient for development of EA. These findings show the critical importance of contrasting cancer and non-cancer outcomes, as otherwise selectively favored traits in pre-cancer tissues will be mistaken for drivers of progression.
-

Nancy Zhang
Detection of genetically distinct subclones and profiling the transcriptomic differences between them is needed for studying the evolutionary dynamics of tumors, as well as for accurate prognosis and effective treatment of cancer in the clinic. For the profiling of intra-tumor transcriptional heterogeneity, single cell RNA-sequencing (scRNA-seq) is now ubiquitously adopted in ongoing and planned cancer studies. Detection of somatic DNA mutations and inference of clonal membership from scRNA-seq, however, is currently unreliable. In this talk, I will describe DENDRO, a new method for subclone detection and DNA mutation profiling using single cell transcriptomic sequencing data. DENDRO utilizes information from single nucleotide mutations in transcribed regions, and accounts for technical noise and expression stochasticity at the single cell level. I will show accuracy evaluations based on spike-in datasets and on scRNA-seq data with known subpopulation structure. Then, I will describe several case studies: We applied DENDRO to delineate subclonal expansion in a mouse melanoma model in response to immunotherapy, highlighting the role of neoantigens in treatment response. We also applied DENDRO to primary and lymph-node metastasis samples in breast cancer, where the new approach allowed us to better understand the relationship between genetic and transcriptomic intratumor variation.
-

Tianjian Zhou
Tumor cell population consists of genetically heterogeneous subpopulations (subclones), with each subpopulation being characterized by overlapping sets of single nucleotide variants (SNVs). Bulk sequencing data using high-throughput sequencing technology provide short reads mapped to many nucleotide loci as a mixture of signals from different subclones. Based on such data, we infer tumor subclones using latent feature allocation models. Specifically, we estimate the number of subclones, their genotypes, cellular proportions and the phylogenetic tree spanned by the inferred subclones. Prior probabilities are assigned to these latent quantities, and posterior inference is implemented through Markov chain Monte Carlo simulations. A key innovation in our method, TreeClone, is to model short reads mapped to pairs of proximal SNVs, which we refer to as mutation pairs. The performance of our method is assessed using simulated and real datasets with single and multiple tumor samples.
-
Michael Metzger
Cancer is normally an evolutionary dead-end—neoplastic cells that arise and evolve within an organism either regress or they kill their host, and the death of the host marks the death of the cancer lineage. However, in some cases, neoplastic cells develop the ability to spread from individual to individual, turning from conventional cancers into clonal contagious cancer lineages. The natural transmission of cancer cells has been observed in two mammals (Tasmanian devils and dogs), and we have found that a leukemia-like disease in soft-shell clams (Mya arenaria) is due to the horizontal spread of a clonal cancer lineage. We also analyzed mussels (Mytilus trossulus), cockles (Cerastoderma edule), and carpet shell clams (Polititapes aureus) and found that the neoplasias in all three of these species are due to independent transmissible cancer lineages. We are currently assembling a reference genome using PacBio sequencing combined with HiC data. Using draft reference genomes, we are investigating genomic changes in the evolution of this unique cancer lineage, including SNPs, structural variation, and copy number variation. In particular, we found a retrotransposon, Steamer, which is expressed and amplified in genomic DNA of the contagious cancer lineage, expanding from 2-10 copies per haploid genome in normal animals to >100 in neoplastic cells. Genomic analysis of cancer samples from isolated clam populations in Maine and Prince Edward Island confirm initial qPCR analysis, and it shows that at least 130 sites are found in cancer cells from both populations. These common sites likely integrated early in the evolution of the cancer lineage, and they have been conserved either as passenger or driver mutations. We also found many insertions that are unique to only one subgroup (494 and 144, unique insertions in Maine and PEI, respectively), showing either continued amplification or deletion after divergence of the cancer in the two populations. These new integration events and other genomic changes have likely played a role in oncogenesis and continued evolution of the cancer with its hosts.
-

David Basanta
Cancer evolutionary dynamics result from the interplay between a heterogeneous tumor and the ecosystem which it inhabits. While beautiful work has shed light on the role of intra tumor heterogeneity and experimental techniques have allowed us to reconstruct the genetic paths that a cancer has followed in a patient, precious little has been done in understanding eco-evolutionary dynamics in cancer. In our group we have use mathematical and computational tools, integrating experimental data and challenged with clinical data, to study the bone ecosystem and its role in explaining the growth and progression of tumors. This will allow us to understand the interplay between the tumor and the bone, how that shapes its evolutionary dynamics and how treatments could be designed that take that into account.
-
David Posada
How and when tumoral clones start spreading to surrounding and distant tissues is currently unclear. Here, we applied a sophisticated evolutionary framework to describe the evolutionary history of a colorectal cancer in time and space. In particular, we have leveraged state-of-the-art approaches from statistical phylogenetics, phylodynamics, and biogeography that allowed us to date the origin of the tumor, to quantify its demography, and to identify the different colonization events that took place. Thus, our analyses strongly support an early monoclonal metastatic colonization, followed by a rapid population expansion at both primary and secondary sites. Moreover, we infer a hematogenous metastatic spread seemingly under positive selection, plus the return of some tumoral cells from the liver back to the colon lymph nodes. This study provides unprecedented detail a picture of the tempo and mode of the tumoral clonal dynamics within a single patient. Importantly, it exemplifies how sound methods from organismal evolutionary biology can be ported to the within-individual level in order to understand complex tumoral dynamics over time and space.
-

Amir Asiaee T.
Cancer is an evolutionary process that can be modeled as a sequence of fixation of genetic alterations throughout the tumor cell population. Each new driver alteration confers a selective growth advantage to the cell and sweeps through the population, which results in clonal expansion. But the order in which accumulating alterations fixate in tumors is not arbitrary and is restricted by the type of advantage that is required to lay the ground for later ones. Perhaps the most famous Bayesian Network model of cancer progression is Conjunctive Bayesian Network (CBN) where the assumption is that all parent alterations must be present in order for a child aberration to occur. The assumption of CBNs is restrictive because a single advantageous hit is usually enough for clonal expansion. We proposed the Disjunctive Bayesian Network (DBN) in which each alteration can occur if at least one of its parents has happened before. DBN generalizes CBN and therefore has a larger search space but we have designed a scalable algorithm to infer DBN from cross-sectional data. I present our specific findings for the order of mutations in melanoma.
-

Kimberly Bussey
What is cancer? It is a fundamental question that still lacks an adequate answer. Cancers or cancer-like phenomena are found across the tree of life in multicellular organisms. The hallmarks of cancer describe the functions a cell or group of cells must express to become a cancerous tumor, including uncontrolled growth, uninhibited mobility, and resistance to cell death. The current paradigm ascribes the acquisition of such behavior to the gradual accumulation of genomic changes. This gene-centric view has been useful up to a point, but it suffers from the problem that most oncogenic changes are neither necessary, sufficient, nor context-independent. Furthermore, such behaviors can be suppressed in a physiologically normal environment.
We propose thinking about cancer as an atavism, in this case the re-expression of single-cell biology in a multicellular context. Cancer is the result of re-deploying single-cell biology in the context of cells that have evolved to be part of a multicellular organism. Under this context, we hypothesize that we can detect evidence of single-cell stress-responses, such as stress-induced mutation (SIM), in cancer genomes. Our work shows that there is evidence of SIM in cancer genomes which has clinical ramifications for both patient survival and treatment approaches.
-
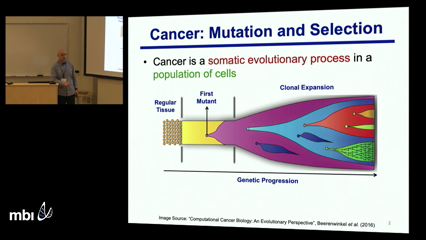
Luay Nakhleh
Intra-tumor heterogeneity, as caused by a combination of mutation and selection, poses significant challenges to the diagnosis and treatment of cancer. Resolving this heterogeneity to identify the tumor cell populations (clones) and delineate their evolutionary history is of critical importance in improving cancer diagnosis and therapy. This heterogeneity can be readily elucidated and understood through the reconstruction of the clonal genotypes and evolutionary history of the tumor cells. Recently introduced single-cell DNA sequencing (SCS) technologies promise to provide the appropriate type of data for resolving intra-tumor heterogeneity. However, inherent technical errors in SCS datasets, due to allelic dropout, cell doublets and coverage non-uniformity, significantly complicate these tasks.
In this talk, I will first describe a maximum likelihood method for inferring tumor trees from SCS genotype data with potentially erroneous and missing entries, under a finite-sites model of evolution. I will then describe a non-parametric Bayesian method that simultaneously reconstructs the clonal populations as clusters of single cells, mutations associated with each clone, and the genealogical relationships between the clonal populations. I will demonstrate the performance of the methods on both synthetic and real data sets.
This is collaborative work with Hamim Zafar, Anthony Tzen, Ken Chen, and Nicholas Navin.
-

Harsh Jain
The average life expectancy of patients diagnosed with glioblastomas is 14 months with treatment. Standard treatment includes the chemotherapeutic drug temozolomide, that works by inducing DNA methylation. However, the BER and MGMT repair pathways efficiently repair the damage caused by this drug, reducing the efficacy of treatment. It has been hypothesized that inhibiting these repair pathways may lead to overcoming chemotherapy resistance. In this talk, I will present a novel mathematical model that captures the effect of chemotherapy on brain cancer cells, and includes detailed mechanisms of DNA damage and repair. The model is extensively parametrized and carefully validated using a wide array of available experimental data. Issues of parameter identifiability are also investigated. A global sensitivity analysis is performed to reveal those parameters most critical in the emergence of chemotherapy resistance. The calibrated model is then applied to predict treatment strategies that are optimized with respect to specific cancer cell phenotypes. A virtual cohort of glioblastoma patients -- each with a heterogeneous tumor -- is created, and a genetic algorithm employed to identify optimal treatment strategies. Our results suggest that patients can be broadly classified into 4 types in terms of these dosage schedules, based on the overall phenotypes of their tumors. Thus, resistance to chemotherapy can be mitigated to a certain extent by using novel dosage schedules, and combining standard treatment with cell-repair enzyme inhibitors.
 Julia ChifmanPanel discussion and workshop wrap-up led by Julia Chifman
Julia ChifmanPanel discussion and workshop wrap-up led by Julia Chifman Julia Chifman, Julia ChifmanPanel discussion led by Julia Chifman
Julia Chifman, Julia ChifmanPanel discussion led by Julia Chifman Laura KubatkoPanel discussion led by Laura Kubatko
Laura KubatkoPanel discussion led by Laura Kubatko Kevin CoombesPanel discussion led by Kevin Coombes
Kevin CoombesPanel discussion led by Kevin Coombes Diego MalloPanel discussion led by Diego Mallo
Diego MalloPanel discussion led by Diego Mallo Marc SuchardPanel discussion led by Marc Suchard
Marc SuchardPanel discussion led by Marc Suchard Russell SchwartzIt has long been recognized that cancer is a process of aberrant evolution, in which clonal diversification and selection result in progression from an initially healthy cell through precancerous and successively more aggressive cancerous states. Genomic studies have increasingly elucidated the finer details of these evolutionary processes and how they act in and vary between cancers, but there are still large gaps in our knowledge and our ability to apply it to translational directions. One key gap is our still-limited ability to predict risk of future cancer progression, e.g., which precancerous lesions are likely to progress to cancer, or which early cancers are likely to threaten the patient, to respond or recur following treatment, or to metastasize. Here, we explore the predictive power for progression outcomes of variations patient-to-patient in evolutionary diversification – i.e., risk arising from patient-specific variation in mechanisms of somatic hypermutability – as compared to the predictive power of selection – i.e., risk arising from patient-specific differences in the spectra of driver gene mutations. We estimate these factors using tumor genome sequence data, combining driver mutation calls with quantitative features derived from tumor phylogeny models and overall mutation burdens to assess in a machine learning analysis how these two classes of evolutionary factors collectively predict future cancer progression. Through application to a set of patient cohorts from the Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC), we show that measures of diversification and selection each contribute complementary and partially orthogonal predictive power relative to one another and to conventional clinical predictors of outcome. The work suggests the importance of better characterizing mechanisms of somatic variation in cancers and their role in cancer risk across diverse progression outcomes.
Russell SchwartzIt has long been recognized that cancer is a process of aberrant evolution, in which clonal diversification and selection result in progression from an initially healthy cell through precancerous and successively more aggressive cancerous states. Genomic studies have increasingly elucidated the finer details of these evolutionary processes and how they act in and vary between cancers, but there are still large gaps in our knowledge and our ability to apply it to translational directions. One key gap is our still-limited ability to predict risk of future cancer progression, e.g., which precancerous lesions are likely to progress to cancer, or which early cancers are likely to threaten the patient, to respond or recur following treatment, or to metastasize. Here, we explore the predictive power for progression outcomes of variations patient-to-patient in evolutionary diversification – i.e., risk arising from patient-specific variation in mechanisms of somatic hypermutability – as compared to the predictive power of selection – i.e., risk arising from patient-specific differences in the spectra of driver gene mutations. We estimate these factors using tumor genome sequence data, combining driver mutation calls with quantitative features derived from tumor phylogeny models and overall mutation burdens to assess in a machine learning analysis how these two classes of evolutionary factors collectively predict future cancer progression. Through application to a set of patient cohorts from the Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC), we show that measures of diversification and selection each contribute complementary and partially orthogonal predictive power relative to one another and to conventional clinical predictors of outcome. The work suggests the importance of better characterizing mechanisms of somatic variation in cancers and their role in cancer risk across diverse progression outcomes. Mary KuhnerBarrett's Esophagus is a neoplastic condition that usually remains stable, but progresses to esophageal adenocarcinoma (EA) in approximately 5% of cases. We used WGS to survey 4 biopsies from 40 EA-outcome and 40 non-EA-outcome Barrett's patients.  These data showed two strongly contrasting patterns. A constellation of genomic-damage indicators including mutations in TP53, copy-number changes, genome doubling, and chromosomal rearrangements were strongly associated with progression to cancer. However, a separate set of features including very high point mutation and indel loads, mutation signature 17, copy-number variation at fragile sites, and positive selection on multiple loci were observed across patients regardless of outcome and showed little to no association with progression. While features in this second group are advantageous in the hostile environment of the Barrett's segment, they are not sufficient for development of EA. These findings show the critical importance of contrasting cancer and non-cancer outcomes, as otherwise selectively favored traits in pre-cancer tissues will be mistaken for drivers of progression.
Mary KuhnerBarrett's Esophagus is a neoplastic condition that usually remains stable, but progresses to esophageal adenocarcinoma (EA) in approximately 5% of cases. We used WGS to survey 4 biopsies from 40 EA-outcome and 40 non-EA-outcome Barrett's patients.  These data showed two strongly contrasting patterns. A constellation of genomic-damage indicators including mutations in TP53, copy-number changes, genome doubling, and chromosomal rearrangements were strongly associated with progression to cancer. However, a separate set of features including very high point mutation and indel loads, mutation signature 17, copy-number variation at fragile sites, and positive selection on multiple loci were observed across patients regardless of outcome and showed little to no association with progression. While features in this second group are advantageous in the hostile environment of the Barrett's segment, they are not sufficient for development of EA. These findings show the critical importance of contrasting cancer and non-cancer outcomes, as otherwise selectively favored traits in pre-cancer tissues will be mistaken for drivers of progression. Nancy ZhangDetection of genetically distinct subclones and profiling the transcriptomic differences between them is needed for studying the evolutionary dynamics of tumors, as well as for accurate prognosis and effective treatment of cancer in the clinic. For the profiling of intra-tumor transcriptional heterogeneity, single cell RNA-sequencing (scRNA-seq) is now ubiquitously adopted in ongoing and planned cancer studies. Detection of somatic DNA mutations and inference of clonal membership from scRNA-seq, however, is currently unreliable. In this talk, I will describe DENDRO, a new method for subclone detection and DNA mutation profiling using single cell transcriptomic sequencing data. DENDRO utilizes information from single nucleotide mutations in transcribed regions, and accounts for technical noise and expression stochasticity at the single cell level. I will show accuracy evaluations based on spike-in datasets and on scRNA-seq data with known subpopulation structure. Then, I will describe several case studies: We applied DENDRO to delineate subclonal expansion in a mouse melanoma model in response to immunotherapy, highlighting the role of neoantigens in treatment response. We also applied DENDRO to primary and lymph-node metastasis samples in breast cancer, where the new approach allowed us to better understand the relationship between genetic and transcriptomic intratumor variation.
Nancy ZhangDetection of genetically distinct subclones and profiling the transcriptomic differences between them is needed for studying the evolutionary dynamics of tumors, as well as for accurate prognosis and effective treatment of cancer in the clinic. For the profiling of intra-tumor transcriptional heterogeneity, single cell RNA-sequencing (scRNA-seq) is now ubiquitously adopted in ongoing and planned cancer studies. Detection of somatic DNA mutations and inference of clonal membership from scRNA-seq, however, is currently unreliable. In this talk, I will describe DENDRO, a new method for subclone detection and DNA mutation profiling using single cell transcriptomic sequencing data. DENDRO utilizes information from single nucleotide mutations in transcribed regions, and accounts for technical noise and expression stochasticity at the single cell level. I will show accuracy evaluations based on spike-in datasets and on scRNA-seq data with known subpopulation structure. Then, I will describe several case studies: We applied DENDRO to delineate subclonal expansion in a mouse melanoma model in response to immunotherapy, highlighting the role of neoantigens in treatment response. We also applied DENDRO to primary and lymph-node metastasis samples in breast cancer, where the new approach allowed us to better understand the relationship between genetic and transcriptomic intratumor variation. Tianjian ZhouTumor cell population consists of genetically heterogeneous subpopulations (subclones), with each subpopulation being characterized by overlapping sets of single nucleotide variants (SNVs). Bulk sequencing data using high-throughput sequencing technology provide short reads mapped to many nucleotide loci as a mixture of signals from different subclones. Based on such data, we infer tumor subclones using latent feature allocation models. Specifically, we estimate the number of subclones, their genotypes, cellular proportions and the phylogenetic tree spanned by the inferred subclones. Prior probabilities are assigned to these latent quantities, and posterior inference is implemented through Markov chain Monte Carlo simulations. A key innovation in our method, TreeClone, is to model short reads mapped to pairs of proximal SNVs, which we refer to as mutation pairs. The performance of our method is assessed using simulated and real datasets with single and multiple tumor samples.
Tianjian ZhouTumor cell population consists of genetically heterogeneous subpopulations (subclones), with each subpopulation being characterized by overlapping sets of single nucleotide variants (SNVs). Bulk sequencing data using high-throughput sequencing technology provide short reads mapped to many nucleotide loci as a mixture of signals from different subclones. Based on such data, we infer tumor subclones using latent feature allocation models. Specifically, we estimate the number of subclones, their genotypes, cellular proportions and the phylogenetic tree spanned by the inferred subclones. Prior probabilities are assigned to these latent quantities, and posterior inference is implemented through Markov chain Monte Carlo simulations. A key innovation in our method, TreeClone, is to model short reads mapped to pairs of proximal SNVs, which we refer to as mutation pairs. The performance of our method is assessed using simulated and real datasets with single and multiple tumor samples. Michael MetzgerCancer is normally an evolutionary dead-end—neoplastic cells that arise and evolve within an organism either regress or they kill their host, and the death of the host marks the death of the cancer lineage. However, in some cases, neoplastic cells develop the ability to spread from individual to individual, turning from conventional cancers into clonal contagious cancer lineages. The natural transmission of cancer cells has been observed in two mammals (Tasmanian devils and dogs), and we have found that a leukemia-like disease in soft-shell clams (Mya arenaria) is due to the horizontal spread of a clonal cancer lineage. We also analyzed mussels (Mytilus trossulus), cockles (Cerastoderma edule), and carpet shell clams (Polititapes aureus) and found that the neoplasias in all three of these species are due to independent transmissible cancer lineages. We are currently assembling a reference genome using PacBio sequencing combined with HiC data. Using draft reference genomes, we are investigating genomic changes in the evolution of this unique cancer lineage, including SNPs, structural variation, and copy number variation. In particular, we found a retrotransposon, Steamer, which is expressed and amplified in genomic DNA of the contagious cancer lineage, expanding from 2-10 copies per haploid genome in normal animals to >100 in neoplastic cells. Genomic analysis of cancer samples from isolated clam populations in Maine and Prince Edward Island confirm initial qPCR analysis, and it shows that at least 130 sites are found in cancer cells from both populations. These common sites likely integrated early in the evolution of the cancer lineage, and they have been conserved either as passenger or driver mutations. We also found many insertions that are unique to only one subgroup (494 and 144, unique insertions in Maine and PEI, respectively), showing either continued amplification or deletion after divergence of the cancer in the two populations. These new integration events and other genomic changes have likely played a role in oncogenesis and continued evolution of the cancer with its hosts.
Michael MetzgerCancer is normally an evolutionary dead-end—neoplastic cells that arise and evolve within an organism either regress or they kill their host, and the death of the host marks the death of the cancer lineage. However, in some cases, neoplastic cells develop the ability to spread from individual to individual, turning from conventional cancers into clonal contagious cancer lineages. The natural transmission of cancer cells has been observed in two mammals (Tasmanian devils and dogs), and we have found that a leukemia-like disease in soft-shell clams (Mya arenaria) is due to the horizontal spread of a clonal cancer lineage. We also analyzed mussels (Mytilus trossulus), cockles (Cerastoderma edule), and carpet shell clams (Polititapes aureus) and found that the neoplasias in all three of these species are due to independent transmissible cancer lineages. We are currently assembling a reference genome using PacBio sequencing combined with HiC data. Using draft reference genomes, we are investigating genomic changes in the evolution of this unique cancer lineage, including SNPs, structural variation, and copy number variation. In particular, we found a retrotransposon, Steamer, which is expressed and amplified in genomic DNA of the contagious cancer lineage, expanding from 2-10 copies per haploid genome in normal animals to >100 in neoplastic cells. Genomic analysis of cancer samples from isolated clam populations in Maine and Prince Edward Island confirm initial qPCR analysis, and it shows that at least 130 sites are found in cancer cells from both populations. These common sites likely integrated early in the evolution of the cancer lineage, and they have been conserved either as passenger or driver mutations. We also found many insertions that are unique to only one subgroup (494 and 144, unique insertions in Maine and PEI, respectively), showing either continued amplification or deletion after divergence of the cancer in the two populations. These new integration events and other genomic changes have likely played a role in oncogenesis and continued evolution of the cancer with its hosts. David BasantaCancer evolutionary dynamics result from the interplay between a heterogeneous tumor and the ecosystem which it inhabits. While beautiful work has shed light on the role of intra tumor heterogeneity and experimental techniques have allowed us to reconstruct the genetic paths that a cancer has followed in a patient, precious little has been done in understanding eco-evolutionary dynamics in cancer. In our group we have use mathematical and computational tools, integrating experimental data and challenged with clinical data, to study the bone ecosystem and its role in explaining the growth and progression of tumors. This will allow us to understand the interplay between the tumor and the bone, how that shapes its evolutionary dynamics and how treatments could be designed that take that into account.
David BasantaCancer evolutionary dynamics result from the interplay between a heterogeneous tumor and the ecosystem which it inhabits. While beautiful work has shed light on the role of intra tumor heterogeneity and experimental techniques have allowed us to reconstruct the genetic paths that a cancer has followed in a patient, precious little has been done in understanding eco-evolutionary dynamics in cancer. In our group we have use mathematical and computational tools, integrating experimental data and challenged with clinical data, to study the bone ecosystem and its role in explaining the growth and progression of tumors. This will allow us to understand the interplay between the tumor and the bone, how that shapes its evolutionary dynamics and how treatments could be designed that take that into account. David PosadaHow and when tumoral clones start spreading to surrounding and distant tissues is currently unclear. Here, we applied a sophisticated evolutionary framework to describe the evolutionary history of a colorectal cancer in time and space. In particular, we have leveraged state-of-the-art approaches from statistical phylogenetics, phylodynamics, and biogeography that allowed us to date the origin of the tumor, to quantify its demography, and to identify the different colonization events that took place. Thus, our analyses strongly support an early monoclonal metastatic colonization, followed by a rapid population expansion at both primary and secondary sites. Moreover, we infer a hematogenous metastatic spread seemingly under positive selection, plus the return of some tumoral cells from the liver back to the colon lymph nodes. This study provides unprecedented detail a picture of the tempo and mode of the tumoral clonal dynamics within a single patient. Importantly, it exemplifies how sound methods from organismal evolutionary biology can be ported to the within-individual level in order to understand complex tumoral dynamics over time and space.
David PosadaHow and when tumoral clones start spreading to surrounding and distant tissues is currently unclear. Here, we applied a sophisticated evolutionary framework to describe the evolutionary history of a colorectal cancer in time and space. In particular, we have leveraged state-of-the-art approaches from statistical phylogenetics, phylodynamics, and biogeography that allowed us to date the origin of the tumor, to quantify its demography, and to identify the different colonization events that took place. Thus, our analyses strongly support an early monoclonal metastatic colonization, followed by a rapid population expansion at both primary and secondary sites. Moreover, we infer a hematogenous metastatic spread seemingly under positive selection, plus the return of some tumoral cells from the liver back to the colon lymph nodes. This study provides unprecedented detail a picture of the tempo and mode of the tumoral clonal dynamics within a single patient. Importantly, it exemplifies how sound methods from organismal evolutionary biology can be ported to the within-individual level in order to understand complex tumoral dynamics over time and space. Amir Asiaee T.Cancer is an evolutionary process that can be modeled as a sequence of fixation of genetic alterations throughout the tumor cell population. Each new driver alteration confers a selective growth advantage to the cell and sweeps through the population, which results in clonal expansion. But the order in which accumulating alterations fixate in tumors is not arbitrary and is restricted by the type of advantage that is required to lay the ground for later ones. Perhaps the most famous Bayesian Network model of cancer progression is Conjunctive Bayesian Network (CBN) where the assumption is that all parent alterations must be present in order for a child aberration to occur. The assumption of CBNs is restrictive because a single advantageous hit is usually enough for clonal expansion. We proposed the Disjunctive Bayesian Network (DBN) in which each alteration can occur if at least one of its parents has happened before. DBN generalizes CBN and therefore has a larger search space but we have designed a scalable algorithm to infer DBN from cross-sectional data. I present our specific findings for the order of mutations in melanoma.
Amir Asiaee T.Cancer is an evolutionary process that can be modeled as a sequence of fixation of genetic alterations throughout the tumor cell population. Each new driver alteration confers a selective growth advantage to the cell and sweeps through the population, which results in clonal expansion. But the order in which accumulating alterations fixate in tumors is not arbitrary and is restricted by the type of advantage that is required to lay the ground for later ones. Perhaps the most famous Bayesian Network model of cancer progression is Conjunctive Bayesian Network (CBN) where the assumption is that all parent alterations must be present in order for a child aberration to occur. The assumption of CBNs is restrictive because a single advantageous hit is usually enough for clonal expansion. We proposed the Disjunctive Bayesian Network (DBN) in which each alteration can occur if at least one of its parents has happened before. DBN generalizes CBN and therefore has a larger search space but we have designed a scalable algorithm to infer DBN from cross-sectional data. I present our specific findings for the order of mutations in melanoma. Kimberly BusseyWhat is cancer? It is a fundamental question that still lacks an adequate answer. Cancers or cancer-like phenomena are found across the tree of life in multicellular organisms. The hallmarks of cancer describe the functions a cell or group of cells must express to become a cancerous tumor, including uncontrolled growth, uninhibited mobility, and resistance to cell death. The current paradigm ascribes the acquisition of such behavior to the gradual accumulation of genomic changes. This gene-centric view has been useful up to a point, but it suffers from the problem that most oncogenic changes are neither necessary, sufficient, nor context-independent. Furthermore, such behaviors can be suppressed in a physiologically normal environment.
Kimberly BusseyWhat is cancer? It is a fundamental question that still lacks an adequate answer. Cancers or cancer-like phenomena are found across the tree of life in multicellular organisms. The hallmarks of cancer describe the functions a cell or group of cells must express to become a cancerous tumor, including uncontrolled growth, uninhibited mobility, and resistance to cell death. The current paradigm ascribes the acquisition of such behavior to the gradual accumulation of genomic changes. This gene-centric view has been useful up to a point, but it suffers from the problem that most oncogenic changes are neither necessary, sufficient, nor context-independent. Furthermore, such behaviors can be suppressed in a physiologically normal environment. Luay NakhlehIntra-tumor heterogeneity, as caused by a combination of mutation and selection, poses significant challenges to the diagnosis and treatment of cancer. Resolving this heterogeneity to identify the tumor cell populations (clones) and delineate their evolutionary history is of critical importance in improving cancer diagnosis and therapy. This heterogeneity can be readily elucidated and understood through the reconstruction of the clonal genotypes and evolutionary history of the tumor cells. Recently introduced single-cell DNA sequencing (SCS) technologies promise to provide the appropriate type of data for resolving intra-tumor heterogeneity. However, inherent technical errors in SCS datasets, due to allelic dropout, cell doublets and coverage non-uniformity, significantly complicate these tasks.
Luay NakhlehIntra-tumor heterogeneity, as caused by a combination of mutation and selection, poses significant challenges to the diagnosis and treatment of cancer. Resolving this heterogeneity to identify the tumor cell populations (clones) and delineate their evolutionary history is of critical importance in improving cancer diagnosis and therapy. This heterogeneity can be readily elucidated and understood through the reconstruction of the clonal genotypes and evolutionary history of the tumor cells. Recently introduced single-cell DNA sequencing (SCS) technologies promise to provide the appropriate type of data for resolving intra-tumor heterogeneity. However, inherent technical errors in SCS datasets, due to allelic dropout, cell doublets and coverage non-uniformity, significantly complicate these tasks. Harsh JainThe average life expectancy of patients diagnosed with glioblastomas is 14 months with treatment. Standard treatment includes the chemotherapeutic drug temozolomide, that works by inducing DNA methylation. However, the BER and MGMT repair pathways efficiently repair the damage caused by this drug, reducing the efficacy of treatment. It has been hypothesized that inhibiting these repair pathways may lead to overcoming chemotherapy resistance. In this talk, I will present a novel mathematical model that captures the effect of chemotherapy on brain cancer cells, and includes detailed mechanisms of DNA damage and repair. The model is extensively parametrized and carefully validated using a wide array of available experimental data. Issues of parameter identifiability are also investigated. A global sensitivity analysis is performed to reveal those parameters most critical in the emergence of chemotherapy resistance. The calibrated model is then applied to predict treatment strategies that are optimized with respect to specific cancer cell phenotypes. A virtual cohort of glioblastoma patients -- each with a heterogeneous tumor -- is created, and a genetic algorithm employed to identify optimal treatment strategies. Our results suggest that patients can be broadly classified into 4 types in terms of these dosage schedules, based on the overall phenotypes of their tumors. Thus, resistance to chemotherapy can be mitigated to a certain extent by using novel dosage schedules, and combining standard treatment with cell-repair enzyme inhibitors.
Harsh JainThe average life expectancy of patients diagnosed with glioblastomas is 14 months with treatment. Standard treatment includes the chemotherapeutic drug temozolomide, that works by inducing DNA methylation. However, the BER and MGMT repair pathways efficiently repair the damage caused by this drug, reducing the efficacy of treatment. It has been hypothesized that inhibiting these repair pathways may lead to overcoming chemotherapy resistance. In this talk, I will present a novel mathematical model that captures the effect of chemotherapy on brain cancer cells, and includes detailed mechanisms of DNA damage and repair. The model is extensively parametrized and carefully validated using a wide array of available experimental data. Issues of parameter identifiability are also investigated. A global sensitivity analysis is performed to reveal those parameters most critical in the emergence of chemotherapy resistance. The calibrated model is then applied to predict treatment strategies that are optimized with respect to specific cancer cell phenotypes. A virtual cohort of glioblastoma patients -- each with a heterogeneous tumor -- is created, and a genetic algorithm employed to identify optimal treatment strategies. Our results suggest that patients can be broadly classified into 4 types in terms of these dosage schedules, based on the overall phenotypes of their tumors. Thus, resistance to chemotherapy can be mitigated to a certain extent by using novel dosage schedules, and combining standard treatment with cell-repair enzyme inhibitors.